Emily Lakdawalla • Dec 30, 2014
Curiosity results from AGU: Methane is there, and it's variable
It's been two weeks since the American Geophysical Union meeting where the Curiosity mission held a press briefing about the detection of methane on Mars. I had intended to write up something on the results while I was at the meeting, but it took a lot longer than I planned. Better late than never!
There were two major announcements. First: Curiosity has definitely, conclusively, observed methane in Mars' atmosphere. There is a very low background level, and then there was a spike to about ten times that, which lasted for a couple of months before disappearing. Second: there are also definitely Martian organic materials in rock samples drilled from the Cumberland site in Yellowknife Bay, but it's still pretty uncertain what that finding means for Martian history just yet. The second result is important but only incrementally different from what I have written on the subject before, so today I'm focusing on the more sensational story: methane on Mars. If you're not familiar with the back story on Mars methane, please read this excellent backgrounder by Nicholas Heavens.
Finding Modern Methane
The press briefing concerned a paper that came out in Science the same day; you can download the Science paper for free here. The presentation was made by Chris Webster, the principal investigator on the Tunable Laser Spectrometer (TLS), one component of the SAM instrument suite. (I wrote an explainer on SAM a while ago.) Unlike the mass-spectrometer part of SAM, the tunable laser spectrometer can only detect and measure three specific gases: methane, carbon dioxide, and water. For carbon dioxide and water, it delivers information on isotopic ratios; for methane, the goal is mostly just to detect it in the first place, although it could do isotopic abundances if there were enough methane around.
Methane was elusive, but it was worth looking for, for the reasons outlined in Nicholas' blog entry. In the first year of the Curiosity mission, the SAM team looked for it six times, clustered in two groups of three, one group while they were near Rocknest (sols 79, 81, and 106) and the other while they were at the Cumberland drill site (292, 306, and 313). One of those measurements, taken during the day of sol 306, was a blip of unusually high abundance, around 5 parts per billion, but it was not repeated and seemed like an outlier.
From those six measurements, they calculated an atmospheric abundance of 0.18 ± 0.67 parts per billion by volume (ppbv). Careful readers will note that the error bars on that measurement go below zero. There's no such thing as negative abundance of methane in the atmosphere; what that value means is that they may have detected no methane at all. All they could say, from that measurement, is to put an upper limit on its abundance, of 1.3 ppbv.
Then something funny happened. On sol 466, just past Cooperstown, they checked the methane level again and found it to be 5 ppbv. They checked again on sol 474, and found 7 ppbv. Again on sol 504, and found 7 ppbv. Still not believing it, they checked again on sol 526 and it was the highest ever measured, 9 ppbv.
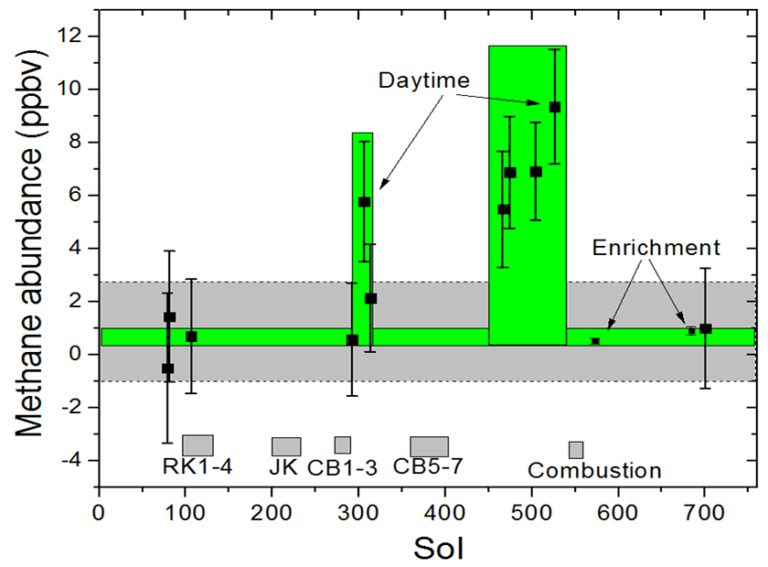
When the SAM team next checked the methane level, on sol 573, seven weeks had passed. They tried a different technique that would earn them much smaller error bars and a more precise reading of what the methane level actually was. SAM's intricate mess of tubing includes a couple of "getters" and "scrubbers," chambers that contain traps for certain gases. They commanded Curiosity to ingest an atmospheric sample and pass the air over a scrubber that removes carbon dioxide, the major constituent of Mars' atmosphere. The air that passes the scrubber is relatively enriched in the minor atmospheric constituents, things like water and argon and, potentially, methane. The enrichment process removes about 96% of the carbon dioxide, multiplying the amount of minor constituents in the remaining air by a factor of 23.
They sent this enriched air into the tunable laser spectrometer and found the methane had nearly vanished; it was at a level of 0.7 ppbv. Crucially, though, this measurement on an enriched sample had much smaller error bars than previous measurements. This time, the error bars no longer reached below zero: it was a definitive detection. They rechecked around sol 700 with two analyses, one of the standard variety and one enriched, and the two agreed, within error: 0.69 ± 0.25 ppbv with the enriched gas, and 0.89 ± 1.96 with the directly ingested, non-enriched air. They had successfully detected a low background level of methane for the first time. They had also documented a puff of methane that lasted about 60 sols, and then disappeared. Given the larger error bars on the earlier, high-methane measurements from sols 474 to 526, there are no statistically significant differences among them, so the SAM team reported an average measurement: 7.19 ± 2.06 ppbv -- ten times the background level.
What does the methane mean?
The low background level of 0.7 ppbv is relatively easy to explain -- in fact, it's expected. Comets, asteroids, and other interplanetary dust that fall continuously onto Mars from space bring with it a certain amount of carbon-rich material. Ultraviolet radiation from the Sun breaks this material into smaller molecules, potentially including methane; according to a study that Webster and coauthors cited, it's estimated that as much as 2.2 ppbv methane could be hanging around in Mars' atmosphere this way, continuously replenished by infalling meteoritic material. Nothing extraordinary is needed to explain the low background level. It's very difficult to measure, so it's pleasing that the SAM experiment worked to do that.
The sudden jump to 5, 7, even 9 ppbv is harder to explain, but not impossible. Methane could be generated underground on Mars through a completely inorganic process called serpentinization. Or maybe there are Mars microbes making it (an extraordinary claim that would require a lot more evidence than a single puff of methane to justify it). Either way, a small geological event -- an impact, a Marsquake, or something -- could release a small puff of methane near Gale crater. On the press panel, Sushil Atreya said "What this is telling us is that Mars is currently active. The subsurface is communicating with the atmosphere." Sushil shared this graphic summarizing the possible sources of methane on Mars.
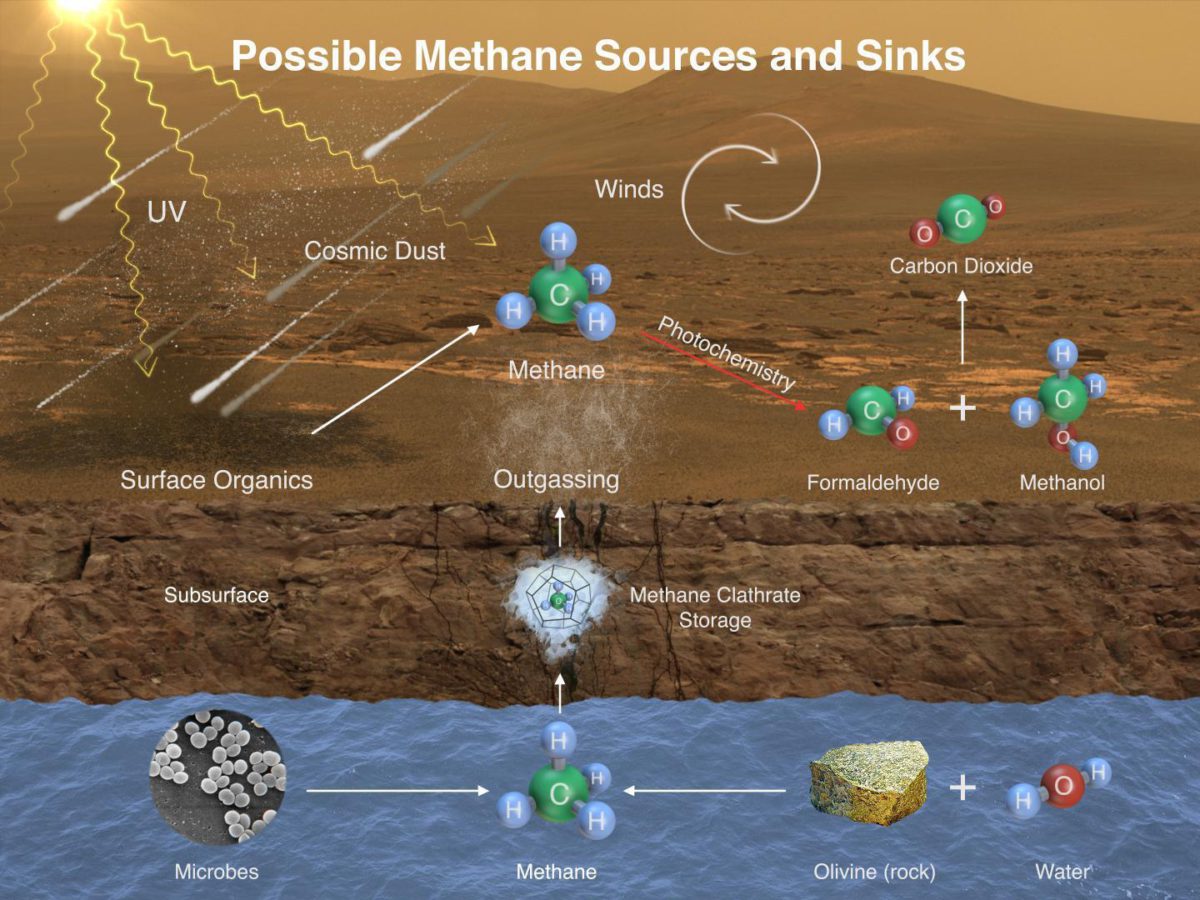
Winds on Mars can quickly distribute methane coming from any individual source, reducing localized concentration of methane. Methane can be removed from the atmosphere by sunlight-induced reactions (photochemistry). These reactions can oxidize the methane, through intermediary chemicals such as formaldehyde and methanol, into carbon dioxide, the predominant ingredient in Mars' atmosphere.Image: NASA / JPL / SAM-GSFC / U. Michigan
A geological event can explain a rapid onset, a puff of methane. When I discussed the methane findings with other scientists at the American Geophysical Union meeting, though, they were having a hard time understanding how it could stick around for two months and then disappear within another two months. If it was just a one-time, local event, why would the elevated levels last two months and then disappear as quickly? A single puff would dissipate quickly; it's hard to understand how some kind of longer-lived natural event would turn on for two months (or more, two is a minimum) and then dissipate completely so rapidly. The SAM team acknowledges this problem in the Supplementary Materials for the paper:
While there is no evidence to suggest an analytical problem despite an exhaustive search for one, such a short-lived elevated methane signal is surprising. Given its transience, an undetected analytical problem cannot be ruled out. Continued measurements of the atmospheric methane abundance, especially using the enrichment procedure, may further establish the reliability of this detection, and possibly of the recurrence frequency of elevated methane concentrations.
By "undetected analytical problem," the team means some unknown issue intrinsic to the SAM instrument, or elsewhere in the rover, can't be ruled out. In the Supplementary Materials to the paper, the authors do try to come up with and eliminate lots of alternative hypotheses for sources for the methane.
Where else could the methane detected by Curiosity be coming from?
The first thing the SAM team has to deal with is the fact that some Florida air leaked into a part of their spectrometer before it launched to Mars. The laser spectrometer has two main parts: A foreoptics chamber (which contains the lasers and some mirrors to guide them) and a Herriot cell (which accepts gas samples and contains mirrors that bounce the lasers back and forth through the cell 81 times before the laser light passes to a detector). The Florida air leaked into the foreoptics chamber. Florida air contains a lot of methane, relatively speaking. They have pumped out the foreoptics chamber and gotten the ambient methane level down to a point where it stays constant. To make a measurement, they first analyze the Herriott cell while it's empty, so any methane they measure is the methane contamination in the foreoptics chamber. Then they pass Martian gas into the Herriot cell and measure that. Then they empty the Herriott cell and measure it again to verify that the methane levels returned to the original level. The methane abundances that they report represent what they measured with the full Herriott cell, minus the readings from the empty cell, canceling out the methane measured in the foreoptics chamber during both readings.
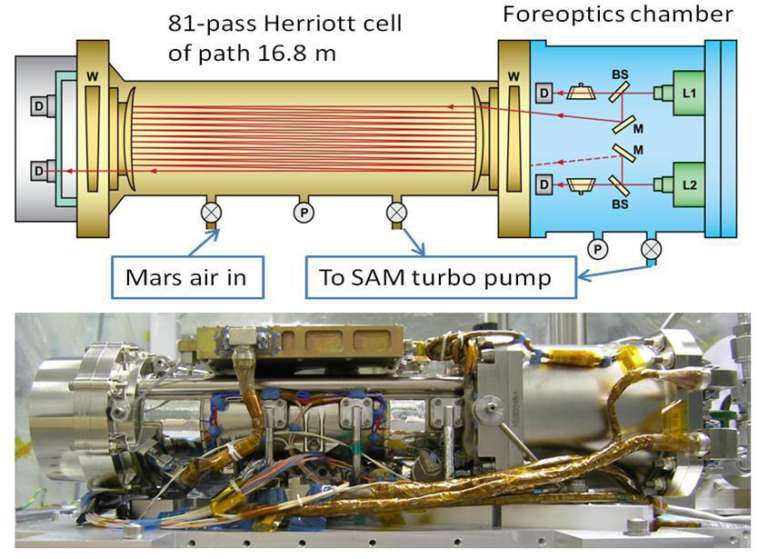
But the Florida air isn't the only possible source of contamination. There's also the problem of the contamination by N-Methyl-N-tert-butyldimethylsilyltrifluoroacetamide (abbreviated MTBSTFA) that has leaked from one of the SAM wet cells and caused all kinds of problems for the SAM team. Whenever SAM cooks a sample in its oven, it sends a portion of the generated gas to the tunable laser spectrometer for isotopic analysis. The evolved gas usually contains quite a lot of methane -- 10 parts per million rather than a fraction of a part per billion -- and the SAM team has concluded that most of this methane comes from the MTBSTFA.
So how can they be certain that the methane that they detect at the few part-per-billion level is actually Martian methane? In the supplementary materials for the paper (which you can download for free here, all 62 pages of it), they go through a number of hypothetical sources of terrestrial contamination, and argue against them. For example, maybe the methane is there because it's leftover from a SAM evolved gas analysis, and the Herriot cell hasn't been completely pumped out. But they show that the levels of methane that they measure -- low or high -- are not correlated with when they did SAM evolved gas runs. Or maybe MTBSTFA contamination builds up on surfaces inside the Herriott cell over time, so it doesn't go away when the cell is pumped out, and then it produces methane when it reacts with the introduced Mars atmosphere for some reason. But they argue that they would detect this contamination as a reduction in the power of the laser, which they don't detect; in fact, if anything, the laser has increased in power (by cleaning off the mirrors inside the Herriott cell) over time. More generally, they just don't see any correlation between when SAM did evolved gas runs and when they saw the low or high methane levels.
They looked for all kinds of other correlations. The high methane levels happened at a time when Curiosity was driving a lot -- actually, during the period of peak wheel degradation. Methane couldn't have come from the wheels themselves -- they're solid aluminum -- but did the wheels somehow make methane by crushing rocks? They looked for, and found, no correlation between how long the rover was sitting around at one spot before a methane reading; the different kinds of terrrain it passed over; or APXS measurements of the chemical content of different rocks. They looked for correlations with season, dust opacity, ground and air temperature, pressure, humidity, water vapor abundance, direction that their inlet was pointing, and background radiation levels. They found a couple of slight possible relationships -- an anti-correlation with water abundance, temperature, and atmospheric opacity, for example -- but all those correlations are wrecked by the low level measured in the first enrichment experiment.
What now?
SAM has, at least, made a good measurement of the background level of methane in the atmosphere at Gale crater, and that is a step forward. And they did detect a transient higher-methane signal -- but it's not at all clear where that came from, or how it vanished so quickly. It's not an entirely satisfactory result; it's still too mysterious to clear up any of the questions about Mars methane that Nick summarized. But the SAM team will continue to do atmospheric analyses as time and power permit, and hopefully they will see another "puff" and find some way to correlate it with events on Mars -- or within the rover. And from above, the Methane Sensor for Mars on Mars Orbiter Mission may be able to make some contribution, someday. When NASA's chief scientist Ellen Stofan visited ISRO in November, one of her goals was to advance NASA-ISRO cooperation that could enable the SAM and Mars Orbiter Mission teams to work together on this problem.
We're missing something. There's something we don't understand here. But that's nothing new, in planetary science. The only thing to do is to keep collecting data and hope it brings us closer to an answer!
Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

