Emily Lakdawalla • Dec 05, 2012
Curiosity update, sol 117: Progress report from AGU
Monday was the big Curiosity day at the fall meeting of the American Geophysical Union. A morning press briefing was followed by an afternoon science session. I traveled to San Francisco briefly just to attend those two events.
The press briefing was mostly concerned with knocking down rumors. It was at the science sessions where I hoped to learn more about the science that Curiosity has been doing. It seemed many others hoped for the same, but the lecture hall provided for the session was absurdly small, and security guards shooed would-be floor-sitters outside for fire safety reasons. So I'm going to put as much as I can into this writeup, for the benefit of the many hundreds of people who didn't make it inside. (How did I make it inside? I've been to enough meetings to predict which sessions are likely to be over-capacity, so I went 20 minutes early.)
I knew that the mission would be starting slowly but I'll admit even I'm surprised that it's sol 119 and the science mission hasn't really begun yet. They're still in the process of commissioning all the instruments and tools. Not much of anything has gone wrong, it's just really complicated, and the complexity has kept everything to a snail's pace.
Since my last update, Curiosity has successfully used the Sample Analysis at Mars (SAM) instrument to analyze several sand samples, so that's one more milestone achieved. The last big hurdle that remains is to drill the first rock and deliver the powder to Chemin and SAM. Until that's done, driving targets are being selected more for engineering than for science reasons.
The bit of science mission that has started is remote sensing of a sort that doesn't care where Curiosity is actually driving. These include environmental measurements by the weather station, radiation detector, and neutron detector. They also include point-and-shoot targeting by the mast-mounted cameras and the Chemcam laser/spectrometer. There was some particularly lovely landscape photography over the Thanksgiving weekend, including the biggest 3D color panorama I've seen yet. This is just a part of it; not all the frames have come down yet. You'll need red-blue glasses. Don't worry, I posted a regular 2D panorama below it. I can't wait to get onto these rocks. And these aren't even the rocks we really came to Mars to see.
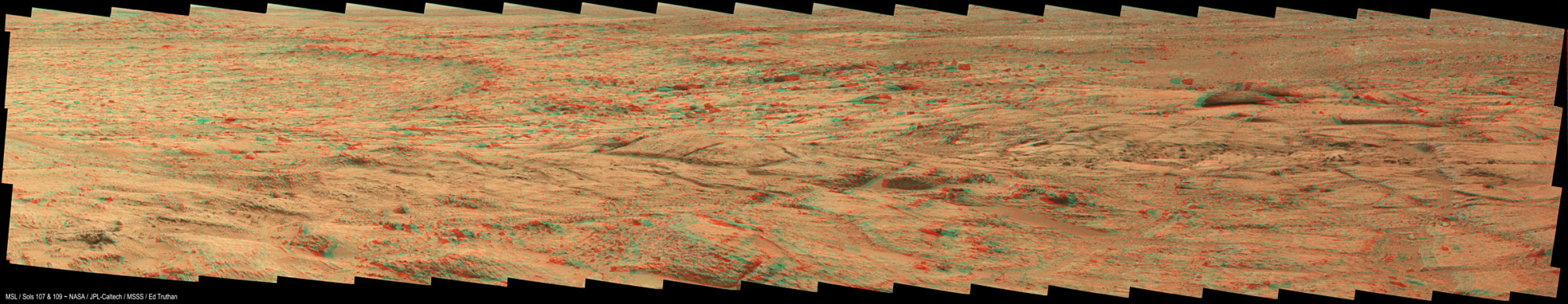

The first presenter was project scientist John Grotzinger, giving an overview. A first impression of the rocks is that they're diverse. There are light ones and dark ones, rounded ones and angular ones, solid ones and holey or "vuggy" ones. One of the more exciting rocks is the conglomerate shown here.
Even that conglomerate is diverse. In some places it's "well-lithified," which is to say it's a pretty hard rock that resists erosion. In other places where Curiosity has seen it, the matrix is much less resistant, so that the river-rounded stones weather out to make a gravelly pavement. The stream that produced these conglomerates was "on the order of ankle- to waist-deep," but they are still working on improving their models to confirm that estimate.
Other interesting rocks include one seen from a distance that had the characteristic "turkey track" texture of a feldspar porphyry, a volcanic rock containing large tabular feldspar crystals. Grotzinger said that the APXS has seen significant levels of potassium in four rocks now. (I wrote about the significance of potassium and feldspar here.)

Staring closely at the soil, Grotzinger noted apparent banding in the Rocknest dune -- stripes of lighter material underlying darker material -- and he also pointed out lustrous, glassy spheres among the Rocknest surface materials, which he proposed were impact spherules.
In the press briefing in the morning, Ken Edgett had had more to say about those Rocknest sands. He used various food similes to explain the different textures. The Rocknest sand, he said, has the grain size of artificial sweetener -- it's finer than table sugar, but coarser than flour. That sand is mantled by a layer of coarser grains, about the size of the salt on soft pretzels. He said they think that the occasional light-colored crystals inside the dune are likely feldspar.
Ashwin Vasavada followed Grotzinger, talking about weather station results. He described how Gale's meteorology was different to previous landing sites'. He said that they have now seen 21 pressure dips lasting about 20 seconds each, the telltale sign of passing "convective vortices." He didn't call them "dust devils" because they don't appear to be lifting dust. Hence, they're mostly invisible.
He said they've only seen one of these vortices visually so far, and pointed out that Gale crater (unlike Gusev) does not contain dust devil tracks. That one dust devil was spotted in a horizon survey on sol 41, Ashwin told me later. It's very subtle, though. It's almost invisible in the JPEG-compressed publicly released images. But we won't have long to wait to see the good-quality science data; Ashwin told me that their goal was to release data from sols 0 to 90 to the Planetary Data System some time in February.
They've been watching the regional dust storm, which has affected the atmospheric opacity (tau) at Gale. Ashwin showed a plot of tau over time. It's been bouncing around 0.6 or 0.7 but reached a high near 1.1 on sol 100. The storm is subsiding, but given Mars' season they expect dust to limit visibility for some time to come. He showed a neat "zenith movie" assembled from Navcam images that had been heavily processed to pull detail out of the bland sky. The animation showed cloud-like features rolling by; Ashwin said it was varying dust.

Ashwin said they don't have any humidity data to report yet, because the sensor is "still undergoing calibration."
David Blake was next onstage, talking first about DAN (Dynamic Albedo of Neutrons). DAN examines the hydrogen content of the ground underneath the rover. The presence of hydrogen is usually attributed to water. It's probably not the kind of water you're thinking of, though; it's water bound within mineral crystals, not liquid water or ice.
Blake reported that they've seen variation in the amount of water in the subsurface along their path. The measurements are mostly not consistent with there being a constant amount of water with depth. Instead, they're best fit by a two-layer model, in which there's an upper dry layer 5 to 30 centimeters thick that contains very little water. ["Very little" is my translation of "less than 0.5% water equivalent hydrogen (WEH)."] Below that dry layer, the ground contains more water (2 to 9% WEH).
Blake then shifted gears to talking about Chemin, the x-ray diffraction instrument that can identify crystalline minerals. He talked about how nice it was to analyze two different samples taken from the same sand dune and get the same results. They have to back mineral abundances out of their results by modeling peaks using a library of spectra of different minerals.
Their models beautifully match their data. Their results on Rocknest: it's a "pretty nice-looking basalt." A basalt containing 43% feldspar; 20.4% olivine (Fo48, if you must know); two different pyroxenes, augite (16.7%) and pigeonite (11.4%); magnetite (1.8%); quartz (1.7%); and less than a percent of anhydrite, hematite, and ilmenite. The presence of pigeonite, he said, means it was an erupted basalt. But this analysis is just for the crystalline material. About half of the sample, he said, was amorphous.
Paul Mahaffy stood up next to talk about SAM results. With SAM, he said, he can help identify at least the volatile component of the amorphous material that Blake was talking about. But the SAM investigations have only barely begun. They've done seven atmospheric analyses, all of them showing less abundant nitrogen than Viking saw. Mahaffy attributed this to their "higher capacity turbomolecular pumps." He said their upper limit on methane abundance is now 5 parts per billion.
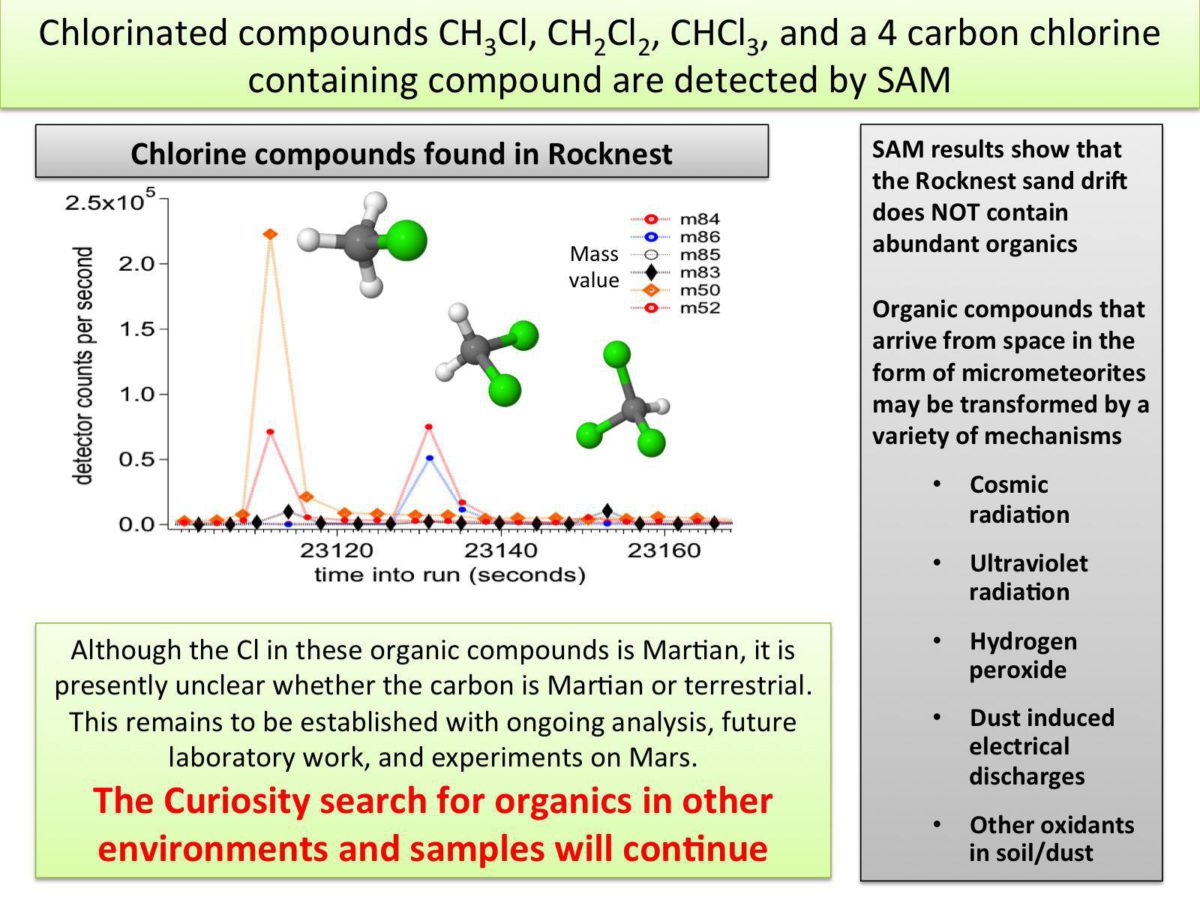
But what everybody was really focused on from SAM was the question of the detection of organic compounds. This story was a challenge for the team to tell at the press briefing. SAM did detect carbon-containing compounds, specifically chloromethane, dichloromethane, trichloromethane, and an as-yet-unidentified four-carbon molecule. But the SAM team cannot yet be certain that the carbon in those compounds came from the Martian soil that they picked up.
Remember that the way SAM works is it slowly heats soil. At different temperatures, the stuff in the sample will decompose into gases. SAM's mass spectrometer identifies those gases and tracks at what temperatures they appeared. For example, a carbonate mineral (something-CO3) will give off carbon dioxide gas (CO2), leaving behind an oxide (something-O). The temperature at which the carbon dioxide gas appears is slightly different for every carbonate, so it may be possible to identify which carbonate was present.
An important point here is that SAM is not directly detecting the constituents of the soil. It's detecting gases that were evolved from the constituents of the soil. Everything it detects has to be made of the same kinds of atoms that started out in the sample cup -- stoichiometry in action -- but the gases are usually new ones, made during chemical reactions among atoms that started out in different minerals.
Mahaffy said they were confident that the chlorine in their observed carbon compounds came from Mars, presumably because there's nothing in the SAM system that could contribute so much chlorine to a sample. But carbon is another story; they can't yet eliminate the possibility that Earthly carbon contamination entered the system somehow. They have no particular reason to suspect that it did, but they can't claim it was Martian until they can prove it wasn't from Earth. And they may not be able to be certain until they pull the trigger on employing an analysis of the Organic Check Material they brought with them.
Evenf the carbon did come from Mars, it wasn't very much carbon. Mahaffy showed some analyses performed on Earth using a testbed version of the SAM instrument. One analysis was performed on an Earthly olivine sand in which Pseudomonas bacteria survive, and the other on a carbonaceous chondrite. These produced rich spectra of a complex variety of carbon compounds. The spectra from the Rocknest sample are nothing like that.
Mahaffy said that the lack of carbon compounds wasn't surprising, though; a wind drift is pretty much by definition exposed to all Mars' elements, including ionizing radiation and triboelectricity. He said that they will be looking for organic materials in much more promising places once they can really start doing science. These include clay minerals, recent impact craters (which would excavate subsurface materials that haven't been exposed to the environment for as long), and the interiors of the most ancient rocks they'll drill into once they can get to the foot of the mountain; or all of the above.
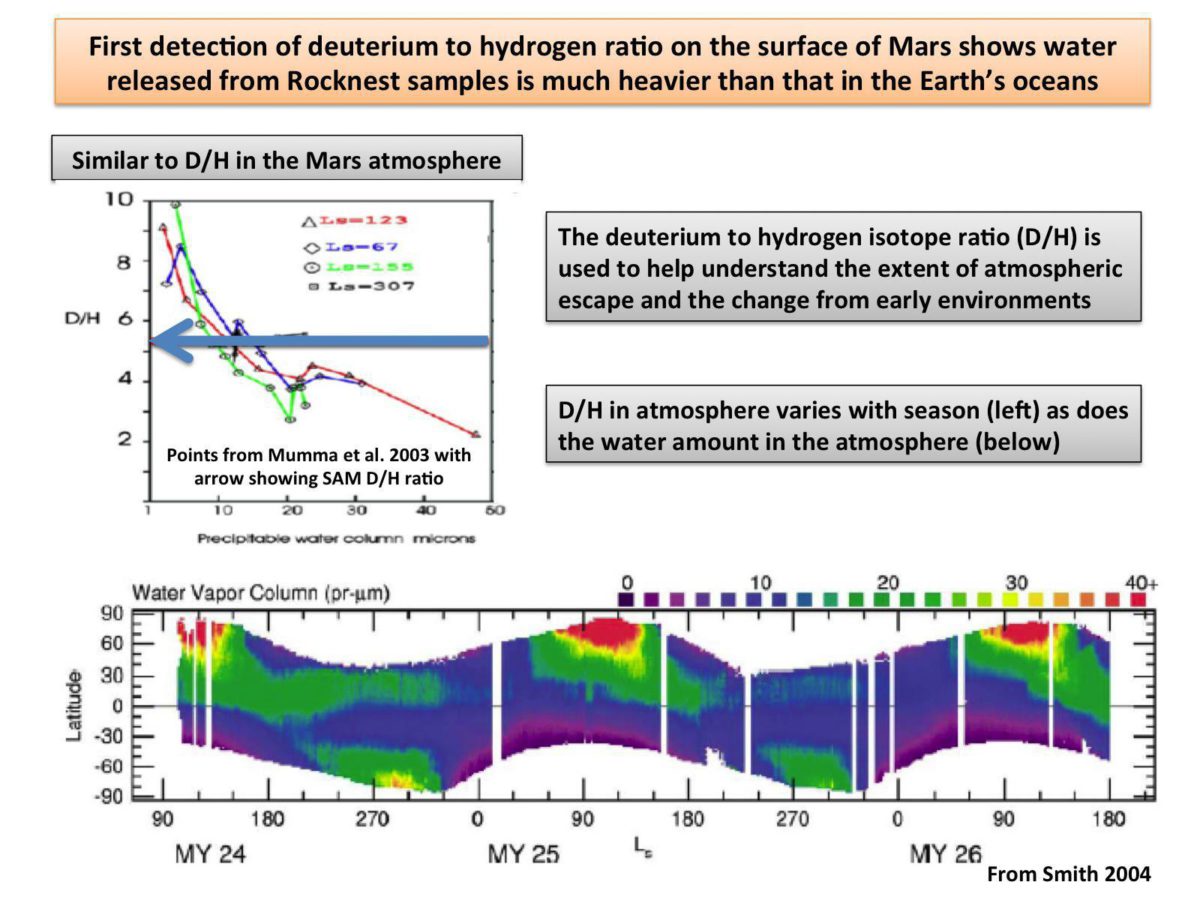
Other stuff they detected included water -- quite a lot of it. There was enough water that they were able to measure the ratio of heavy hydrogen (deuterium) to regular hydrogen, a quantity known as the D-to-H or D/H ratio. They found the D/H ratio to be five times that of Earth. That's significant because it suggests Mars has lost a lot of its atmosphere to space. Now, this is no surprise. But Mahaffy and also Grotzinger were really excited about the D/H measurement.
They hope to be able to make the same measurement in the ancient rocks at the foot of the mountain, and thereby study how and when the atmosphere was lost. I asked him if the hydrogen in those rocks would definitely be old hydrogen (which would preserve an ancient D/H ratio) or if it could be new hydrogen? Mahaffy said they should be able to disentangle that. If new hydrogen has replaced old hydrogen, it should evolve off the sample at a relatively low temperature. Hydrogen coming off the sample at a high temperature would likely be old.
They saw carbon dioxide releases at a couple of different temperatures, likely indicating the presence of a carbonate, though not calcium carbonate, Mahaffy said later. At Barbara Cohen's prompting I asked if Chemin also saw a carbonate, and if so, which one? But Chemin hasn't seen it, for two possible reasons. Chemin only sees major minerals; if there's much under a percent of any mineral, they won't see it. And the carbonate could very easily be amorphous, in which case it'd be invisible to Chemin.
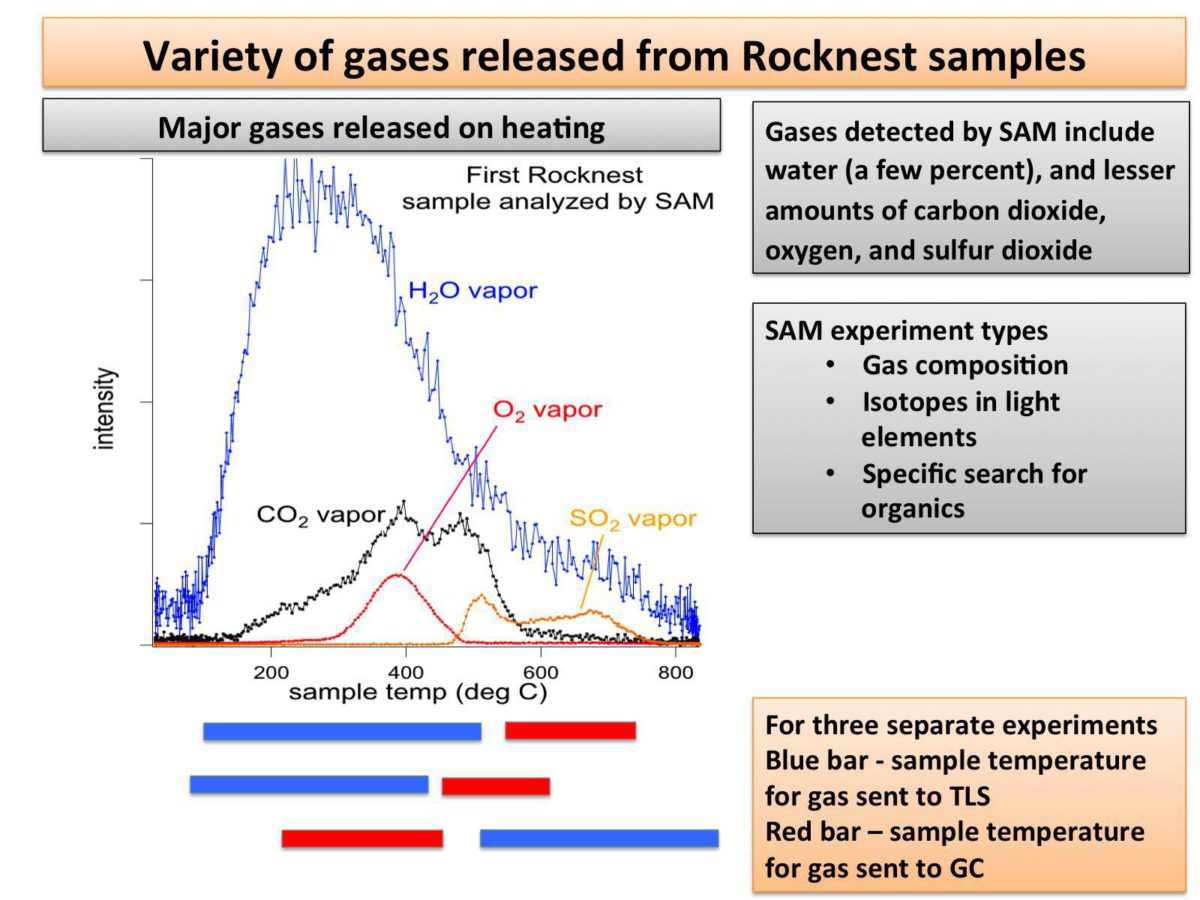
They saw molecular oxygen, both the standard kind and the heavy kind (with oxygen-18). The chlorine compounds, coupled with the release of oxygen, implied to Mahaffy that the chlorine had originally been present as perchlorate ion, likely in the form of calcium perchlorate. Perchlorate was also detected by Phoenix; it is nice to corroborate that. They also saw hydrogen sulfide and sulfur dioxide, possibly representing a sulfate of some kind. And they also saw trace amounts of a material that they definitely did bring with them, a volatile compound stored in their wet chemistry cells, abbreviated MTBSTFA. Mahaffy said they're working on understanding how that contributes to the carbon dioxide they're detecting.
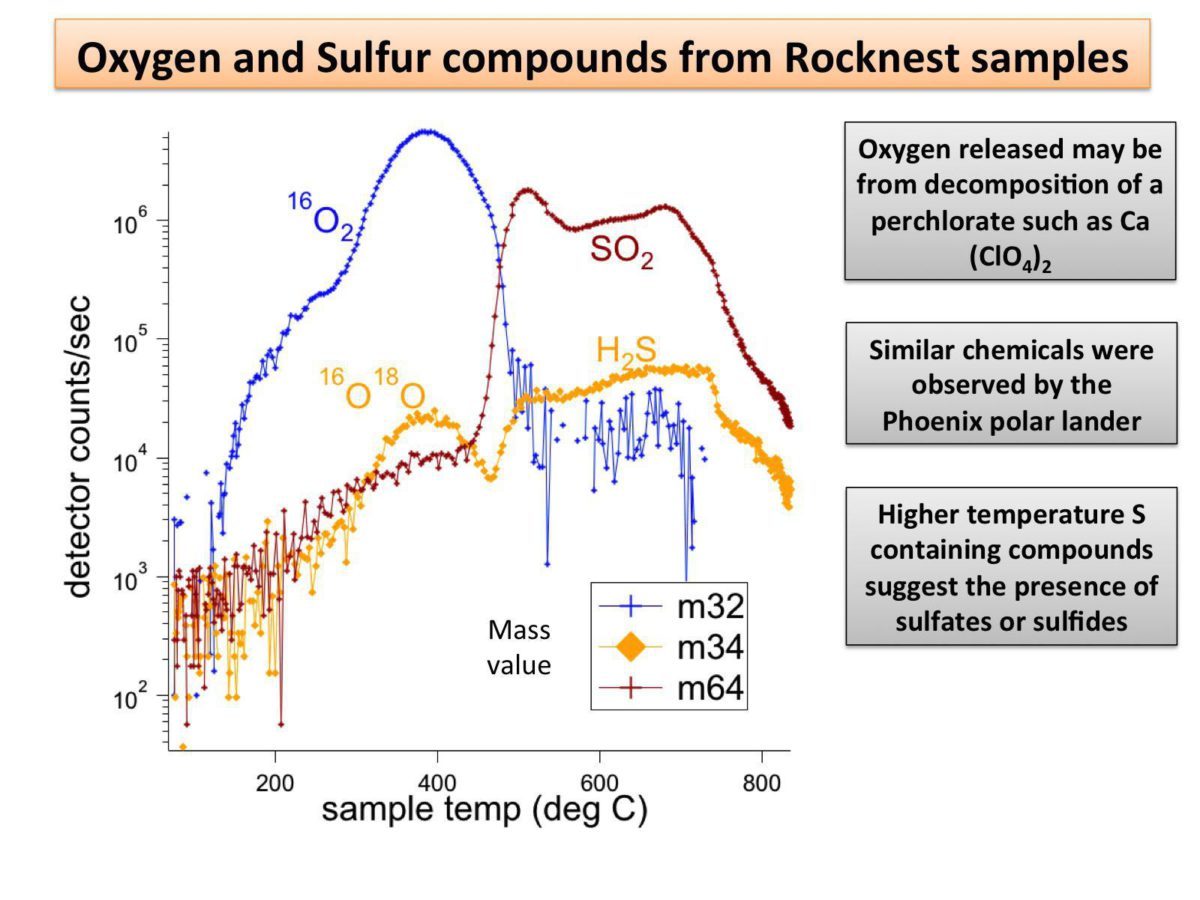
SAM has now ingested four samples of the Rocknest sand. They aren't repeating exactly the same experiment on all of them. They're heating the sand each time, but they're varying the experiment to send different fractions of gases taken at different times to the laser spectrometer, thoroughly characterizing the material.
The results so far look good; it's nice to know the machine is working. But analyzing a sand drift is not what either SAM or Chemin were sent to Mars to do. Both Mahaffy and Blake looked forward to actually examining rocks, which will be much more interesting scientifically.
Next up was Don Hassler, presenting on the radiation detector. His was actually the first instrument to start taking science data, well before the cruise. He said that although there have been measurements of the space radiation environment before, these were the first taken from a position buried inside a spacecraft. That's clearly relevant to future human exploration of Mars. The spacecraft can provide shielding, but cosmic radiation impinging on spacecraft can also produce secondary radiation that might be different from the triggering type.
Hassler said that Curiosity's spacecraft shell provided a similar amount of shielding to that provided by the Space Station (an effective shielding, he said, of 28 g/cm2). The highest-energy galactic cosmic rays weren't cut very much, but the solar contribution was substantially reduced. He compared the radiation detector's data to counts of high-energy protons made during the same period by the ACE mission and found that the spacecraft provided significant shielding to Curiosity particularly during solar events. Looking at total dose rates, the radiation dose Curiosity received during its cruise to Mars would present a problem for humans, 1.90 ± 0.28 mSv/day. Since landing, the dose equivalent rate has been a much lower 0.7 ± 0.17 mSv/day, a rate that would be tolerable.
Next up was Roger Wiens talking about ChemCam results. They've noticed a significant transition in the surface material along their traverse. Near where Curiosity landed, through sol 50, the surface materials were coarse (a grain size around 7 millimeters). Since sol 50, the grain size has been much finer.

This transition was reflected in ChemCam measurements. Prior to the transition, Chemcam was seeing wildly variable silica abundances in the rocks, probably due to some of these grains being feldspar -- hit a grain and you get high silica, miss a grain and you get low silica. After the transition to smaller grain size, ChemCam measurements more accurately reflect whole-rock compositions, and the silica abundance has "settled down."
In general, the soil composition has been consistent with that observed by the older rovers. It's the pebbles and conglomerate rocks that are richer in alkali elements.
And those are all my notes from the session. In general, the data look lovely but it's very early in the mission to have learned much. Because they're still commissioning these instruments, the stuff they're selecting for study is not being picked based on scientific interest. Quite the contrary, they are looking for the most ordinary material they can find. "Ordinary" might not be quite the right word -- how can something be "ordinary" on another planet? -- but the point is that they want to sample material whose composition they think they understand pretty well. A good result is finding that the material matches expectations. At this stage, we can't distinguish good surprises (discovery of something new and fascinating) from bad surprises (instrument isn't working properly), so surprises are to be avoided.
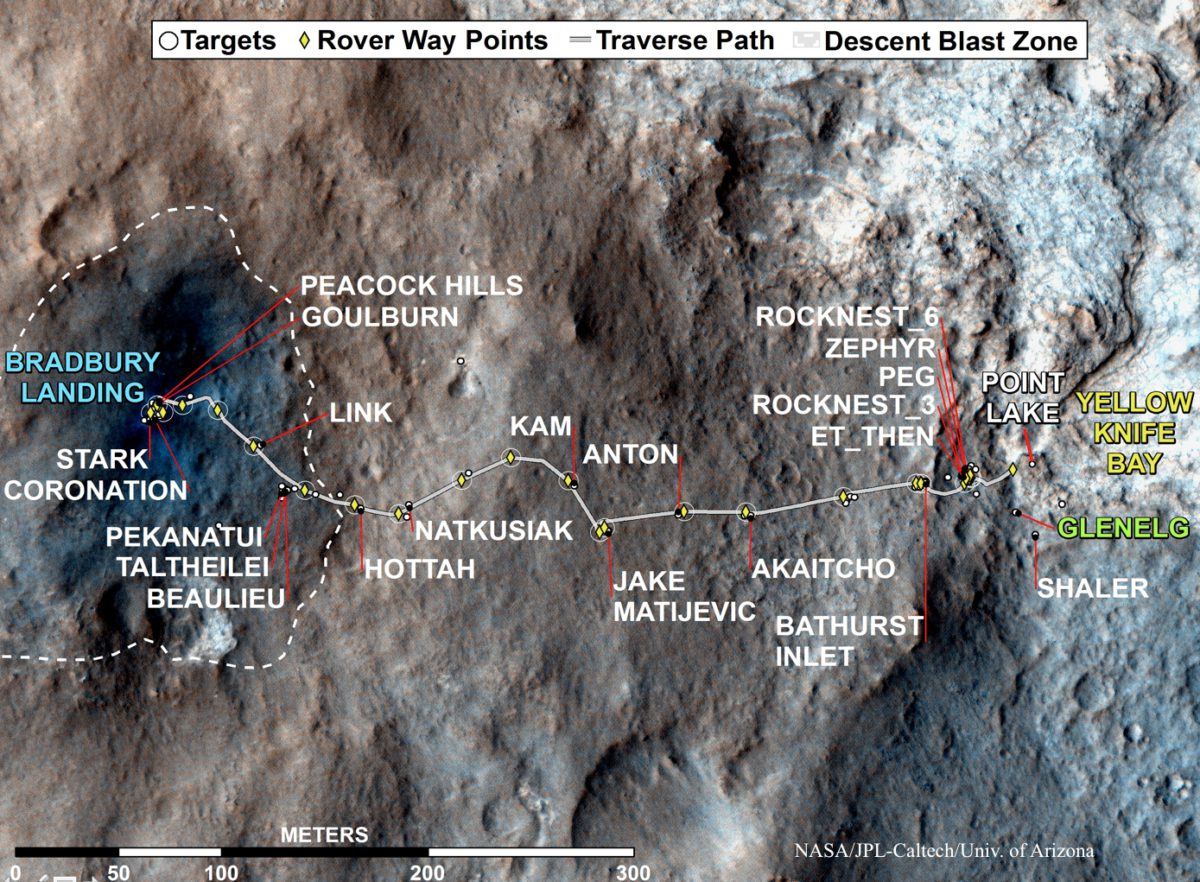
We're on the edge of Glenelg now: hopefully, Grotzinger said, we'll drill before the holidays, and start the trek toward the mountain after the New Year.
If all of this wasn't enough for you, here's Ashwin giving a 1.5-hour presentation to the SETI Institute on the first four months of Curiosity's mission.
Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

