Seven Rasmussen • Feb 15, 2022
How we use starlight to look for alien life
In movies, humans encounter aliens in a variety of ways. They come to us, like in Ted Chiang’s “Arrival,” or we go to them, like Ridley Scott’s “Alien,” or perhaps some resolute scientist detects a radio signal from a nearby star, like in Carl Sagan’s “Contact.” Out of these three scenarios, a radio signal seems like the most likely — no one has to invent interstellar spaceships, for example, or worry about how to breathe in someone else’s atmosphere.
“Contact” comes pretty close to getting it right. Stars and their planets are always emitting signals for scientists to decode. But, unlike the technological messages in science fiction, these signals are created by nature — and we can detect them with a process called spectroscopy.
The signature of life
The kinds of natural signals astronomers look for are called biosignatures: gases — or some other substance — that can only be made by living creatures.
Methane is a common example of a biosignature because the only way it can stick around in an atmosphere is if it’s constantly being produced by something — otherwise, it will react with other chemicals and turn into a different gas. Life like what we see on Earth often produces a lot of methane. In this way, the content of a planet’s atmosphere can tell us whether it might host life.
The Search for Life
Learn how The Planetary Society works to elevate the search for life as a space exploration priority.
Now let’s zoom out a little bit. When we look at a planetary system beyond our own, 99% of the time all we can see is its star, even with the best camera on the biggest telescope. This is just because planets are much smaller and fainter than their stars. So how exactly can we tell what a planet’s atmosphere is made of when we can’t even see it?
Spectroscopy is the study of light and all its colors, and it works because a planet is still emitting light even if we can’t resolve it with our cameras on Earth. In the last 20 years, astronomers have developed several clever tricks to use spectroscopy to search for biosignatures.
Spectroscopy 101
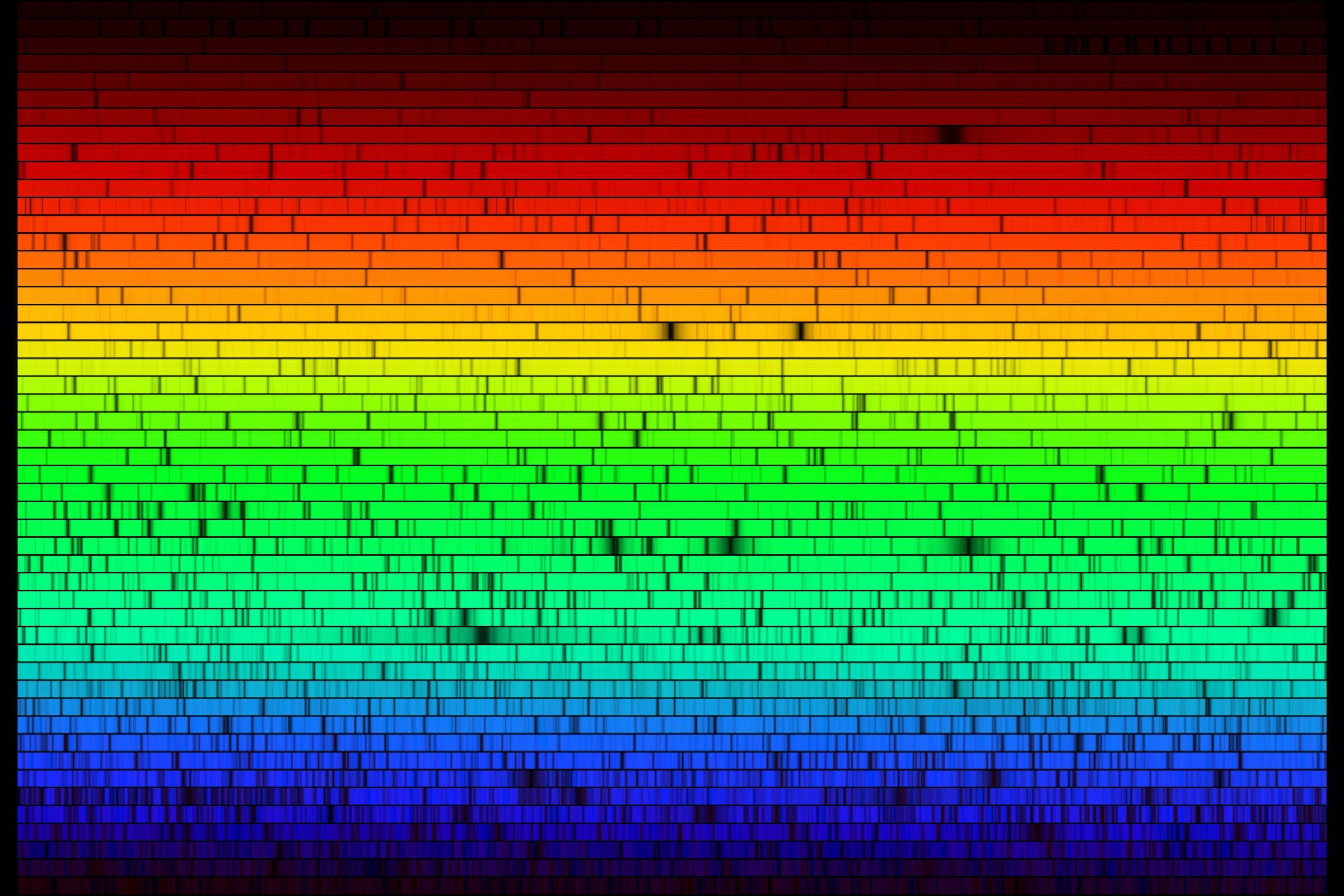
The key concept of spectroscopy is that light from stars, which usually appear to be white, is actually composed of the full electromagnetic spectrum, from radio to X-ray. When white light passes through a glass prism, the light spreads out into a rainbow of colors. Why? Because different colors bend at different angles when they pass through a material like glass, with red bending the least and purple the most, as demonstrated below by prog-rockers and inadvertent science educators Pink Floyd.
If you look at a star through a prism, you will notice something unusual: the rainbow it produces has some dark patches in it. This is because stars are made largely of hydrogen and helium — atoms that “catch” very particular colors of light and prevent them from leaving the star.
How does that work?
It all has to do with the structure of the atom — protons and neutrons live in the nucleus, or center of the atom, and electrons whiz around the nucleus like hyperactive children.
If we continue this analogy, let’s think of starlight as a bunch of different colored balls flying past the kids, trying to get past them. The hydrogen atom’s single electron is like a child who will only catch balls of very specific colors: light purple, dark purple, dark blue, light blue, and red.
Now, when we collect all the balls that got through and put them through our prism, we will be missing those colors that the child caught. The same principle applies to helium’s two electrons, which have different color preferences than hydrogen.
In fact, the higher up in the periodic table you go, the more electrons an atom has (more kids to catch more balls!), and the more dark lines it creates. Every atom has a set of dark lines, which is unique to it, known as a spectral signature. The full set of colors and dark patches is called a spectrum.
When we look at a planet's spectrum, it can tell us which atoms and molecules are present, including potential biosignatures like methane. But how do we get a planet's spectrum on its own, without interference from its host star?
Motion Capture
When something luminous moves, all of the colors in its spectrum shift a little bit. This is known as the Doppler effect, and it works the same way as hearing an ambulance siren pass by, when the pitch of the sound seems to get higher and then lower. Light waves from an approaching object are compressed toward the blue end of the spectrum, and light waves from a receding object are stretched toward the red. So when a planet orbits its star, its spectrum is either red- or blue-shifting, while the star’s spectrum does not shift.
Astronomers take advantage of this fact by taking several spectra in a row of a star that hosts a planet. Let’s consider our methane spectrum and say that, to our camera, it has a big, dark line at pixel number one. By the time the sequence ends, the same line may have shifted over to pixel number six. But the star’s spectrum didn’t shift at all.
That means it can be subtracted out, leaving behind just the planet’s spectrum, and some noise. This whole process is called emission spectroscopy.
That’s it! This method of seeing the planet’s spectrum through the star’s is our biggest key to the search for life. As bigger and better telescopes like the recently launched JWST move us toward the future of looking at habitable and inhabited planets, exoplanet spectroscopy will be our eyes — and someday, those eyes will make the discovery of a lifetime.
Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth