Emily Lakdawalla • Jan 12, 2012
Evaporites on Titan
The following was posted in December as a Planetary Geomorphology Image of the Month, a monthly Web feature provided by the Planetary Geomorphology Working Group of the International Association of Geomorphologists, and I thought it'd be of interest to you. --ESL
by Jason W. Barnes
Assistant Professor of Physics, University of Idaho
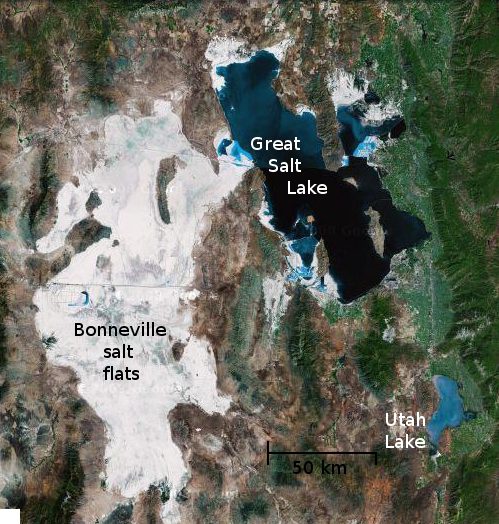
Evaporites form on planetary surfaces when dissolved chemical solids precipitate out of saturated solution as their liquid solvent evaporates. Until recently, they were known to exist on only two planets: Earth and Mars. On Earth there are a variety of evaporite constituents including carbonates (CaCO3), sulfates (CaSO4), and halides (NaCl), progressing in order of increasing solubility. NASA's rover Opportunity discovered evaporitic deposits on Mars that are primarily composed of sulfates. These are different from Earth's evaporites due to a highly acidic formation environment.
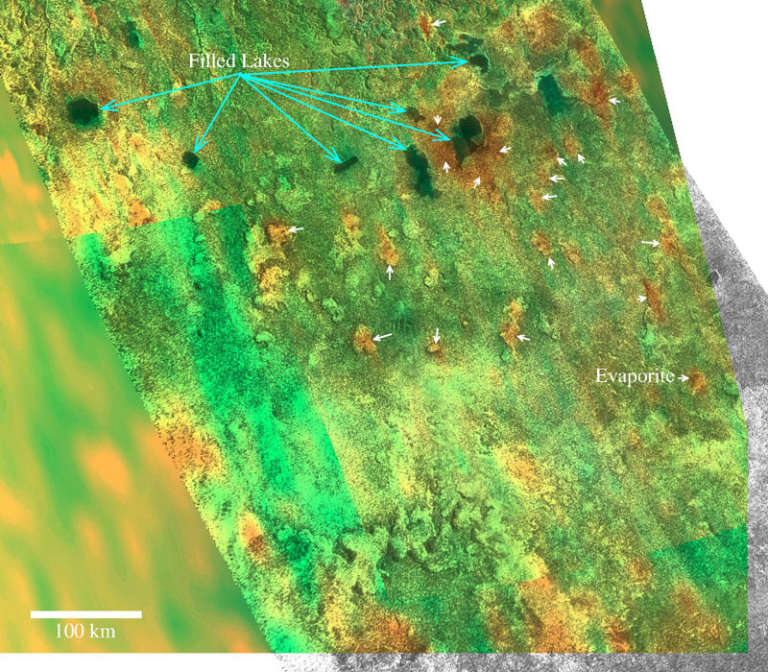
A third planetary instance of evaporite has now been discovered in an exotic location: Saturn's moon Titan. Being so far from the Sun, Titan has a low surface temperature of 90°K (-183°C), just warmer than liquid nitrogen. Hence all of Titan's water is permanently frozen. However, methane on Titan plays the same role that water does on Earth and Mars. Titan has methane clouds, methane rain, methane rivers, and methane lakes and seas.
Therefore, the evaporites on Titan have an unusual nature compared to those on rocky planets. Instead of water being the solvent, on Titan the solvent is methane. And instead of salts being the solute, on Titan organic molecules derived from ultraviolet photolysis of methane dissolve in rain, surface, and ground liquid. Those organics precipitate out of lakes when the liquid methane solute evaporates, becoming evaporite.

Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

