Mike Brown • Mar 06, 2013
Sea Salt
This blog entry was originally posted in three parts on Mike Brown's blog and is reposted here with his permission. --ESL
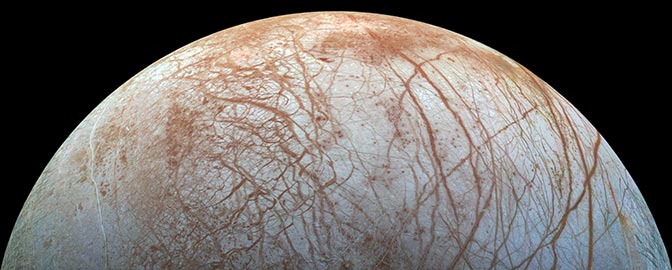
Ever wonder what it would taste like if you could lick the icy surface of Jupiter’s Europa? The answer may be that it would taste a lot like that last mouthful of water that you accidentally drank when you were swimming at the beach on your last vacation. Just don’t take too long of a taste. At nearly 300 degrees (F) below zero your tongue will stick fast.
The composition of the surface of Europa has been hotly debated since the Galileo mission attempted to make detailed measurements more than a decade ago. Galileo’s tool for measuring the composition was spectroscopy -- looking at the sunlight that reflects off of the surface of Europa and seeing which molecules leave characteristic fingerprints in that reflected sunlight. It’s a powerful technique, one that led to the initial discovery of water ice on the Galilean satellite, the discovery of frozen methane on distant bodies like Eris and Pluto and Makemake, and is even used on the Earth to map out mineral deposits for potential exploitation.
The data from Galileo were exciting. While for years the surface of Europa has been known to be dominated by frozen water, Galileo saw that something was mixed in with the water, and, at first, that something was thought to be salts. Not “salt” as in the sodium chloride of your table salt, but more generically “salts” as in “things that dissolve in water and stick around when the water evaporates.” You can imagine why that idea is exciting: if the surface is covered by things-that-dissolve-in-water-and-stick-around-when-the-water-evaporates that strongly implies that Europan ocean water has flowed on the surface, evaporated, and left behind salts. Whatever else is in the ocean is left behind, too. Microbes, fish, whales, you name it, all sitting on the surface waiting to be seen. But there’s more. If the ocean makes it to the surface it probably means, in general, that the ocean and in surface might be in intimate chemical contact. And the surface is rich in energetic molecules (for reasons we’ll discuss below), so that means that energy could be being deposited into the ocean. And if you have water and you have energy? Well, you can guess where this train of thought leaves you.
But wait. Further analysis of the spectrum of Europa suggested that an equally good explanation is that -- rather than salts -- Europa is covered in sulfuric acid. While it sounds strange, it is actually perfectly natural. The ices of Europa are constantly bombarded by sulfur that initially came from the volcanoes on Io (okay, this part is sort of strange). In fact, if you look at Europa you can quickly see exactly where the sulfur lands. There is a bullseye of red on the side of Europa that faces the bombardment.
And, in addition to making the surface rain, when the sulfur slams into the icy surface of Europa, the ice and sulfur react to make sulfuric acid. You can imagine the chemistry. Water is H2O. Sulfur is S. Add the two and it is perhaps not a surprise that you get H2SO4, or sulfuric acid. There are two things that are interesting about sulfuric acid on the surface of Europa. First, it means that the surface and the ocean might be completely disconnected. And, second, it is inevitable. Sulfur does bombard Europa. Sulfuric acid must be on the surface. So does that mean there are no salts? No whales? Well, no, not necessarily.
The real problem is that with the spectrograph that was on the Galileo spacecraft it was nearly impossible to tell the difference between salts and sulfuric acid. While the two sound very different, they do one thing the same: absorb water. And, once they do absorb water, when you look at the fingerprint of the reflected light with your spectrograph, what you mostly see is that water. There are differences, but you can only detect them if you look at that fingerprint finely. It’s as if you were looking at real fingerprints and you could tell that two were similar because they generally had loops and whorls in the same places, but you didn’t have a good enough view of the finger print to be able to discern all of the individual patterns that would tell you whose hand that finger belonged to. The Galileo spectrograph could tell there was adsorbed water, but it really couldn’t tell who it belonged to, so, in the absence of concrete evidence, most of the discussion of the composition of the surface of Europa has been dominated by what people think should be there, rather than what the inconclusive data show. The consensus view that has settled in is that Europa has both salts and sulfuric acid on its surface. The sulfuric acid is dominant on the half of Europa that is bombarded by sulfur, while the salts dominate elsewhere. And the main types of salts that are thought to dominate the surface of Europa are sulfates. “Sulfate” simply means SO4, so a sulfate salt is something like sodium sulfate -- Na2SO4 -- or, one that has been mentioned quite frequently for the surface of Europa, magnesium sulfate, MgSO4. Magnesium sulfate sounds like a fairly random chemical for the surface of Europa, but it is not, for two reasons. First, it’s a relatively common and important salt even on the earth. If you are in the hospital and your doctor does a blood test on you and fears you are low on magnesium, you will be given a solution of magnesium sulfate.
Second, it is, perhaps, something we might expect to find on Europa. Experiments from more than a decade showed that if you take a meteorite – which might represent the types of rocks that the interior of Europa is made out of – and you put it in a pot of hot water for a while and then measure what is in the water when you are all done, one of the things that you are likely to get out of the meteoritic rock is magnesium sulfate. The argument here, though, is a bit circular. Magnesium sulfate is expected, something is seen that can’t quite be identified, it is declared to be magnesium sulfate, and people say -- Look! This is probably true because it agrees with the earlier experiments.
It’s all an interesting story, but wouldn’t you like to know what is really going on? I would. While no spacecraft is going to be in the vicinity of Europa for at least a decade, in the thirty years since the Galileo mission was first conceived and designed telescopes on the Earth made advances that were – truly – inconceivable at the time. And while it’s hard to beat flying up close to learn what is going on, we can now see things from the Earth even better than we could from Galileo. It has been clear for a while now that it has been time to head out to a big telescope and figure out what the real story is.
One of the biggest problems with trying to figure out what is on the surface of Europa was that the spectrograph on the Galileo spacecraft didn’t have a very fine view of the reflected light coming off the surface. The analogy I used earlier was that Galileo was looking at fingerprints where you could only discern the rough pattern and not the individual ridges. You couldn’t use those fingerprints to know for sure who had smudged your crystal, though you might be able to rule out some people and you might become more suspicious of others.
There are two main reasons that the views from Galileo were not as fine as we would like. First, Galileo was old when it arrived at Jupiter. Serious work began on the spacecraft in 1977, and with typical delays and atypical space shuttle accidents, it was finally launched, via a space shuttle, in 1989. Even the trip to Jupiter took longer than initially planned -- the shuttle accident spawned new rules which required the use of a less powerful rocket to launch Galileo from the orbiting shuttle -- so Galileo could not go directly to Jupiter but instead had to get gravity sligshots off of Venus and Earth before finally heading towards Jupiter and arriving in 1995, nearly twenty years after construction began. It was old on the first day it took data at Jupiter. (It was intentionally crashed into Jupiter in 2002 to prevent, among other things, an accidental crash into Europa, which would clearly disturb the whales.) Not surprisingly, the old technology was not as good as current technology in seeing precise spectral fingerprints.
The second reason that the spectrograph on Galileo didn’t see as fine detail as one might like is that, being on a spacecraft, it was extremely tightly constrained in how big and heavy it could be. Even in 1977, the technology existed for finer detail, but it didn’t exist in a form that you could package into the small size needed to head to Jupiter.
Today, the technology for finer detail is readily available. But, sadly, we don’t have anything new headed to Jupiter any time soon. What to do? Head to the Keck telescope.

The Keck telescope is no longer particularly new, either (it is celebrating its 20th birthday this month!), but telescopes, unlike [most] spacecraft, get new instruments all the time. And, even better, the instruments can be the size of a room. Keck has a relatively new (and extremely large) spectrograph – called OSIRIS – which has a ~40 times finer view of the spectrum than the spectrograph on Galileo did. There is, however, one serious disadvantage to the Keck telescope: no one has yet figured out a way to fly it to Jupiter. Again: what to do? Luckily, astronomers (and, earlier, spooks) invented an answer here. Turn on your adaptive optics.
Telescopes on the surface of the Earth are limited in the details they can see in space by the smearing caused by the turbulence of the Earth’s atmosphere. A star no longer looks like a single point of light in the sky, but instead looks like a big smeary point of light in the sky. More importantly, Europa doesn’t look like a little world with interesting features, but instead looks like, well, a big smeary point of light in the sky. Adaptive optics changes everything. I had what I think of as a very nice description of just how adaptive optics works in my book (that would be How I Killed Pluto and Why It Had It Coming, in case you missed it) which I will post next week for people interested in the details, but, for now, think of it as a magical device which makes the Earth’s atmosphere go away and allows the telescope a pristine view of the sky (if you’re a spook, you are excited by this, because it allows very pristine view of enemy satellites as they zip across the sky). A pristine view of the sky with no atmosphere in the way is still not as good as flying to Jupiter, but, from a monster telescope like the Keck telescope, it’s enough to get a nice regional map of Europa.
So we flew off to Hawaii to spend four nights at the Keck telescope taking spectra of Europa. Four nights is just enough time to watch Europa as it circles Jupiter and presents all of its different faces to us. Four nights should give us a better spectral view of Europa than we have ever had before. Four nights could -- fingers crossed -- answer the questions about the composition of the surface of Europa that remained elusive even after up close scrutiny from the Galileo spectrograph.

How does Europa look from the Keck telescope when you turn on the adaptive optics system? Here’s a good example. A picture of Europa from the Keck telescope, showing where there is a lot of water ice (blacker) vs. where there is stuff that has water but is definitely not water ice (redder), compared to a Galileo picture of Europa. Ok, Ok, the Galileo picture is definitely better, but guess what? We now have a really good spectrum at every point in the Keck picture of Europa. Pictures are nice and you can learn a lot by studying them, but specra. Spectra! That’s where we really learn what is going on. So what is going on?
The first thing that you notice when you look at a spectrum of Europa -- from the Earth, from a spacecraft, it doesn’t really matter – is the ice. Ice is everywhere. The spectrum of ice is a very distinctive looking thing, with a quickly recognizable pattern of regions where the sunlight reflects strongly from the surface and regions where there is less reflectance (and remember the regions here means spectral regions, which means, essentially, we stare at one small spot on the surface, put the light through a prism to spread it all out, and see which colors of the rainbow are present and which are absent. In our case our rainbow is in infrared light that your eye can’t see, but the idea is still the same).
The second thing that you notice -- and this is so noticeable that it was recognized by astronomers at telescopes on the ground long before Galileo arrived and long before adaptive optics gave pristine views -- is that on the reddish trailing side of Europa the water ice looks funny. Remember, the water ice looks funny because it is not really water ice, but water that is adsorbed on to something else. It looks similar to water ice, but different.
The first thing we did with the global spectral maps of the surface of Europa was to figure out where there was water ice and where there was something else. And we made a map that looks like this:

Though that little blip (“spectral feature” we call it) is tiny, and we didn’t immediately know what caused it, we did know one thing immediately. It was not caused by sulfuric acid. Just this little bit of information is critical: something is on the surface of Europa that is not an obvious by-product of sulfur ions hitting water ice. This result already answers one of the questions we set out to address: is the reddish non-water-ice stuff on the trailing side of Europa all sulfuric acid? No. No, it’s not. While much of the reddish material is likely still sulfuric acid (remember: it has to be there), there is definitive evidence now that there is something else there.
But we can do more. Much much more. We can try to figure out what chemicals on Europa’s surface cause that new spectral feature. The first thing we did was to carefully map out where we see that new spectral feature. We had found it by looking at the reddish material, but where else might it be? The answer, which surprised us greatly, is: nowhere else. This new spectral feature, which doesn’t look like any expected chemical species that we would expect from sulfur ions bombarding water ice, appears on the parts of the surface where sulfur ions are bombarding water ice, and nowhere else. Weird. Very weird.
We went to the laboratory to try to reproduce that new spectral feature. (I should mention, at this point, that “we” is me and my friend and colleague Kevin Hand, who is a scientist at JPL specializing in Europa, among other things). We tried normal things (dissolve salts in water, freeze them, look at the spectrum), we tried slightly unusual things (freeze Epsom salt, crush it, sieve out big particles, medium particles, tiny tiny partucles, take spectra), we tried truly strange things (freeze Drano, grind it up, take a spectrum). In all of these experiments we only found one material with the new spectral feature. And that material is one that had been suspected all along: epsomite, a magnesium sulfate salt with water bound to it. Remember, epsomite was one of the favorite salts that people thought had been detected on the surface of Europa. But also remember that the actual evidence was quite thin and that it was more of an inference based on what people thought the ocean composition might be. The argument was, essentially: we seen non-water-ice stuff on Europa which might be salt, we expect the oceans to have magnesium and sulfate, thus those salts must be magnesium sulfates, and, with the not-very-precise data that we currently have, they could well be magnesium sulfates. I will admit that I always found this line of reasoning unconvincing, so magnesium sulfates were the last thing that we expected to find. But there they were, just as had been predicted for 15 years.
Two parts of this story don’t add up. If magnesium sulfates are coming from the internal ocean and making it to the surface of Europa, why do they only do it on one side? And why does that one side happen to be the side with sulfur raining down on top of it? Suspicious, no?
And, finally, there is one more clue. More than 15 years ago, when I was a freshly-minted Ph.D. looking around the solar system, I discovered anatmosphere of sodium atoms surrounding Europa. A few years later (when I was at Caltech and finally could use the mighty Keck telescope on my own!) I found potassium alongside the sodium. All of these years, we have assumed that the sodium and potassium come from the salts on the surface of Europa and that these salts get knocked off the surface of Europa (by the radiation, among other things) where we can see them in the atmosphere. So the question you might want to ask is: hey, what about magnesium? If there are magnesium salts, shouldn’t magnesium get kicked up into the atmosphere? More than ten years ago, my then summer undergraduate research fellow, Sarah Horst, looked for magnesium using data from the Hubble Space Telescope. She didn’t see any. “Huh,” we both said, and put the data in a drawer and didn’t think about it for most of the last decade. When we detected the magnesium sulfates a few months ago, I realized it was time to dust off those results. I called Sarah, who had gone on to graduate school, finished, and is now a postdoctoral fellow at the University of Colorado, and said “let’s write that paper.” We did, and it came out recentlyin the Astrophysical Journal Letters. In that paper we showed that magnesium is undetectable in Europa’s atmosphere, and, for this to be true, magnesium can’t be dominant.
All of the pieces of the puzzle are now in place. From everything that I just told you, Kevin Hand and I constructed a hypothesis. I’ll call it a hypothesis here, because much of it is a story that we constructed to fit all of the available evidence, but it is a story that we do not yet have the data to verify. But here it goes:
It seems unlikely that ocean-derived magnesium sulfates would only cover one side of Europa. It seems even more unlikely that that one side would also happen to have sulfur raining down. Sulfates. Sulfur. Hmmmm. So what if, instead, the sulfate (that’s SO4) is formed by sulfur slamming into ice. And the irradiation breaks about the magnesium salt and created magnesium sulfates. That is exactly what happens to make sulfuric acid on Europa’s surface, but now magnesium is involved instead. In what form is magnesium originally? We don’t know. We’ll call it MgX.
The leading side of Europa, which appears to have no magnesium sulfates, is, presumably, instead covered by MgX. But not too much of it, because there is more sodium and potassium than magnesium, it appears. So, most likely, the surface has NaX and KX, too.
But what is the mysterious chemical X?
Here, we have no direct evidence at all, so we have to resort to inference. This inference is not much better than the one about sulfates that I complained about earlier, but it is the best we can do, so we are going to do it. Examining the chemistry of the solar system and asking ourselves about likely oceanic components and ruling out sulfates really leaves one major suspect: chlorine. Which would make the important salts on the surface NaCl, KCl, and MgCl2. You’ve heard of NaCl before. You call it salt. The others are in our oceans here on earth, too.
This chlorine hypothesis – the “sea salt hypothesis” we like to call it – if true, implies major difference in the ocean chemistry with chlorine being an important component of a chemically reduced ocean. But we’ll talk about chemical implications later.
(We made this cool picture for the press release. How exactly it is supposed to demonstrate our results I am not certain, but it's cool looking, no?)
For now these results have a few key conclusions:
- The surface of Europa has magnesium in some salt form. Salt from the ocean gets on the surface. If salts get on the surface, other stuff gets on the surface.
- While people have speculated for a while about drilling down to the ocean to find out its composition, it doesn’t sound like that is important. If you want to know the composition of the Europa ocean, go lick the surface.
- When you do, we suspect that it may taste surprisingly familiar to those who have recently ingested an accidentally mouthful of sea water.
- While no one is going to be licking the surface of Europa anytime soon, the great power of modern giant telescopes at Earth will be used increasingly to take spectral fingerprints of increasing detail to finally understand the mysterious details of the salty ocean beneath the ice shell of Europa.
What happens next? We look for chlorine, I think. The existence of chlorine as one of the main components of the non-water-ice surface of Europa is the strongest prediction that this hypothesis makes. We have some ideas on how we might look; we’re working on them now. Stay tuned.
While this post has been extra long (longer than the scientific paper, I think, since there was a lot of background to get through), you now have enough background and inside knowledge that you might even consider, for fun, reading the scientific paper on which all of this is based. You can find it right here.
Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth