Sarah Hörst • Aug 26, 2013
Probing Titan's Atmosphere
By now I hope that everyone has seen some of the spectacular images of the Saturn system (and especially Titan!) from the Cassini-Huygens mission. I must admit I am always a little bit jealous of the scientists who work on these images because it is so easy to get the public excited about their discoveries. It does not take much explanation to elicit a "wow" response to the radar images of Titan's lakes. Instead the measurements that often make my heart race are taken by instruments that reveal Titan in ways that our eyes cannot see.

The types of measurements we make can be divided into two categories: remote sensing and in situ. Remote sensing measurements can be thought of as measurements where we can see but we cannot touch. A good example are the spectacular images take by the HiRISE camera on the Mars Reconnaissance Orbiter. In-situ measurements, which are much more rare in planetary science, allow us to "touch." Anything we do from the Earth is remote sensing. When we send spacecraft they often carry both types of instruments, and Cassini-Huygens is no exception. Being able to acquire both types of measurements is particularly important for the study of planetary atmospheres.
Remote sensing techniques, like ultraviolet and infrared spectroscopy, allow us to see how light interacts with molecules in an atmosphere. Each molecule does this in its own unique way and this allows us to quantify the amount of a molecule in an atmosphere by looking at the light that gets absorbed, emitted, or transmitted. The first molecule ever detected in Titan's atmosphere, and indeed our first solid evidence that Titan had an atmosphere, was methane (CH4), which was discovered by Gerard Kuiper in the 1940s. Methane has a very characteristic way that it interacts with light. This behavior makes it one of the molecules that we can, and have, detected in exoplanet atmospheres as well. People are often surprised that we can measure the composition of exoplanet atmospheres -- they often think that what they hear in the news is just a guess. It is mind-boggling, but true, that photons can travel over unimaginable space and time to tell us that long ago, in a faraway land, they interacted with a methane molecule.
When the Voyager spacecraft flew through the Saturn system in the early 1980s, they used infrared spectroscopy to detect a whole suite of molecules in Titan's atmosphere: acetylene (C2H2), ethane (C2H6), propane (C3H8), hydrogen cyanide (HCN), carbon dioxide (CO2), et cetera. These measurements were our first glimpse into the amazing complexity of the chemistry occurring in Titan's atmosphere. But the Voyager data covered just one moment in time and only gave us information about one region of the atmosphere. Different wavelengths of light allow us to sense different parts of the atmosphere and different molecules because the different photon energies require different numbers and types of molecules to absorb them. So we really want ALL of the photons ALL of the time.
But even if we had all of the photons all of the time, we wouldn't be able to see everything. Some molecules do not interact strongly with light. Some examples include argon and even the main constituent of Titan's atmosphere, nitrogen (N2), whose abundance was not really well known until Cassini-Huygens. Also, very heavy molecules are more difficult to detect with remote sensing.
The way a molecule interacts with light has a lot to do with the different ways that they can bend, wiggle, and rotate to get rid of the energy they get from photons. (For a good time, ask someone who does infrared spectroscopy to demonstrate the way that propane can bend and vibrate.) In general the more atoms it has, the more ways it can wiggle. And each different wiggle has a different characteristic wavelength. The more ways it can wiggle, the more spread-out the signal from the wiggles gets. Think of the difference between a spotlight and a whole bunch of flashlights spread far apart. The more spread-out the signal is, the more difficult it is to detect. So in general, larger molecules are harder to detect from remote sensing.
If we are lucky, we get the opportunity to send in-situ instruments like mass spectrometers so that we can detect these kinds of molecules that are hard to detect from remote sensing. Mass spectrometers do not look at how molecules interact with light. Instead, they can separate molecules by mass. One drawback of mass spectrometry is that it can only tell us what the atoms are in a molecule (how much carbon, nitrogen, hydrogen, et cetera) but not how they are arranged. Infrared spectroscopy really tells us that molecule you are seeing is benzene, whereas mass spectrometry would tell us that we have C6H6.
Mass spectrometry is particularly useful in regions where the atmospheric density is low and there are fewer molecules to interact with light, which makes it harder to "see" them. So not only do we want all of the photons all of the time, but we also want all of the mass spectrometry all of the time.
Back to Cassini-Huygens. The orbiter and the probe carried a veritable army of instruments to characterize the gas phase composition of Titan's atmosphere. During the Huygens probe's descent, the Gas Chromatograph/Mass Spectrometer (GCMS) it carried measured the composition at the very bottom of Titan's atmosphere (the troposphere), which is a difficult region to measure from remote sensing. Cassini carries three remote sensing instruments for atmospheric measurements: the Composite Infrared Spectrometer (CIRS), which measures the composition of the stratosphere; the Ultraviolet Imaging Spectrograph (UVIS), which looks higher in a region called the mesosphere; and the Visible and Infrared Mapping Spectrometer (VIMS), which is really meant for the surface but since the pesky atmosphere gets in the way it is able to give us information about the atmosphere as well. Cassini also carries in-situ instruments because the spacecraft sometimes flies through the very top of Titan's atmosphere, where it can use an instrument called the Ion and Neutral Mass Spectrometer (INMS) to measure the composition of the upper atmosphere (which we call the ionosphere or the thermosphere depending on our purpose). If you were reading the tweets that inspired this blog post, INMS is the instrument I referred to as taking "om nom nom" measurements.
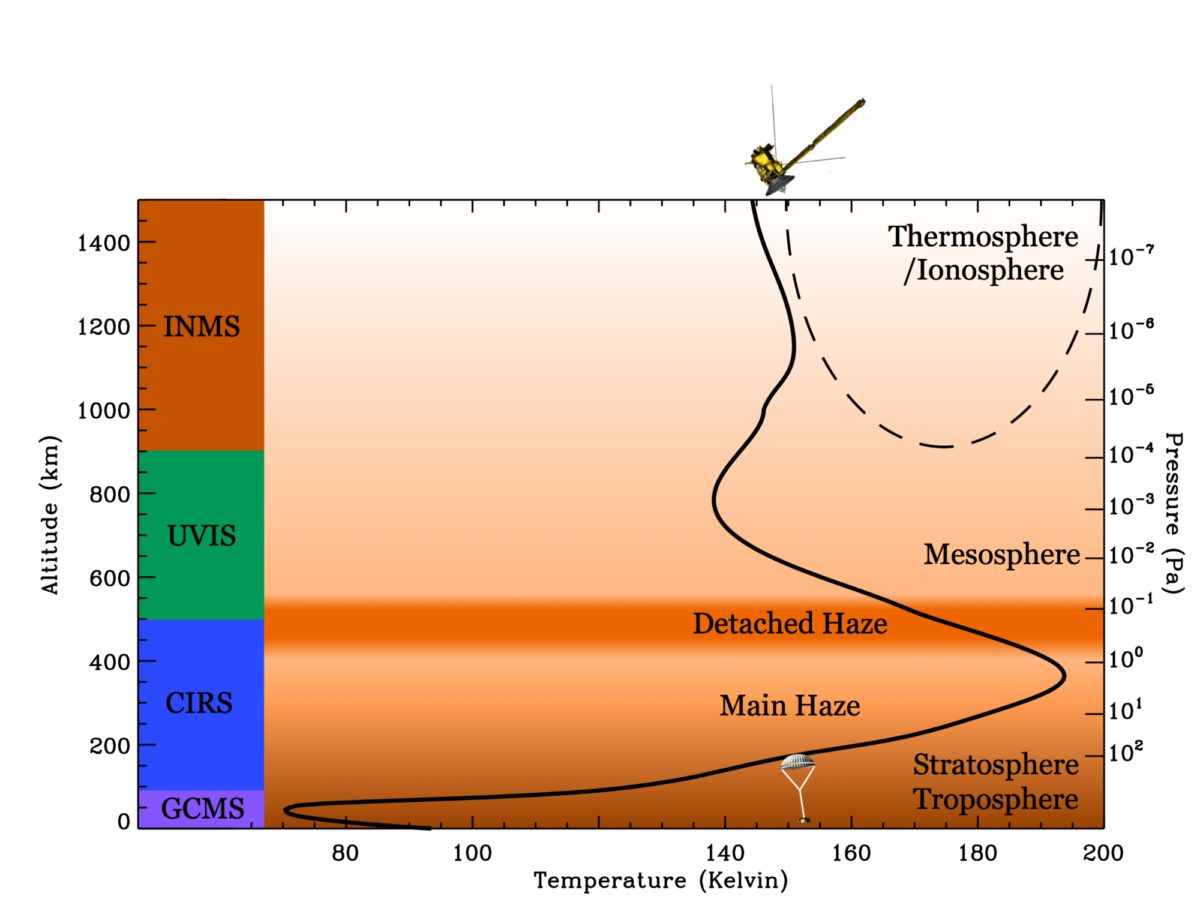
Now we have measurements that cover the whole atmosphere and allow us to detect a much larger number of molecules that we could see from Voyager or can detect from Earth. Not only that, but Cassini has been in orbit around Saturn for nine years (nearly a third of a Titan year) and we have measurements from almost 100 Titan flybys. Note, though, that we do not have 100 sets of measurements from each instrument. The instruments require different orientations to do their measurements, and the scan platform which would have allowed the instruments to make measurements at the same time was "descoped" -- that is, it fell victim to budget cuts. Instead, through a lot of careful planning, different instruments take priority on different flybys. These numerous flybys have allowed us to learn about the composition in different places (latitude, longitude, altitude) in Titan's atmosphere and also have given us information about how the atmospheric composition changes over time, especially seasonally.
That was a rather long introduction to what will be a much too brief overview of two amazing things that we have learned about Titan's atmosphere so far.
First, I mentioned that we are getting information now about how the composition of Titan's atmosphere varies with season. This is particularly striking in the stratosphere, where measurements from CIRS show us that the abundances of some molecules change dramatically with season (to the point that some can only be detected in the winter), while others do not vary at all. Cassini arrived at almost the same point in a Titan year that Voyager observed (one Saturn year or almost 30 Earth years between missions :/): northern hemisphere winter. Both Voyager and Cassini detected enhancements of some molecules at northern high latitudes. Why does this happen?
Well, we know now that at the winter pole, air descends from higher up in the atmosphere. We also know that the complex molecules in Titan's atmosphere are made in chemical reactions that start when photons and/or energetic particles break up nitrogen (N2) and methane (CH4). Each kind of molecule has a different story though. Some molecules, like benzene (C6H6), are made almost solely by ion chemistry occurring very high in Titan's atmosphere, while other molecules, like ethane (C2H6), are produced throughout the atmosphere. Where they are made, and how much of them there are, is defined by the reactions required to make them, which are in turn driven by abundances of building blocks (reactants). The abundance of reactants is defined by the energies of photons and energetic particles.
When you move air from the upper atmosphere to the lower atmosphere -- which is what is happening at Titan's winter pole -- two things can happen. First, you bring molecules to a colder region of the atmosphere, which can make some of them condense from a gas to a liquid or solid. Second, you may move parcels of air that contain higher abundances of molecules that were made in the upper atmosphere to regions of the atmosphere where they were much less abundant. By measuring the composition of Titan's atmosphere, we can actually see the way that the air is moving (the atmospheric dynamics!).
Since Cassini has been in orbit for almost a third of a Titan year now, we have been able to watch the composition of the atmosphere change, as Titan has moved out of northern hemisphere winter, through spring, toward summer. As the abundances near the north pole are beginning to return to "normal", we are beginning to see an increase in these molecules at the south pole.
We've also seen changes in the atmosphere at wavelengths that are visible to our eyes. The Voyager spacecraft and Cassini, when it first arrived to the Saturn system, observed a north polar "hood". The north polar hood is region that appeared darker than the rest of Titan's orange haze. Last year Cassini watched in real time as a vortex formed at the south pole, a phenomenon we'd never seen before because we have never been in the Saturn system for Southern hemisphere winter.
People are still working to understand the formation of the vortex. For me one of the most striking things about the formation of the vortex is that it is a visible reminder that other planets, just like our own, are not fixed. They are ever-changing systems that respond to external and internal changes. As I said on Twitter, seasonal changes are particularly exciting because they act as natural "experiments" on a planet to help us test our understanding of the atmospheric chemistry and dynamics. It is one thing to be able to explain one set of data taken at one point in time, but it is quite another to understand variations with time, and eventually to be able to predict what will happen in the future. In fact, the change in abundance of the molecules at the south pole was predicted. But it did not happen quite when it was predicted, so people are working to figure out what piece of understanding we are still missing. Titan does not give up its secrets without a fight.
Now a brief story about the upper atmosphere. I briefly touched on some discoveries from Cassini about the upper atmosphere when I mentioned the incredibly heavy ions discovered in Titan's upper atmosphere using data from the Cassini Plasma Spectrometer (CAPS). Those measurements really deserve an entire post of their own, but rather than talk about that incredible discovery, I'm going to tell you a little bit about the smaller molecules measured by the Ion and Neutral Mass Spectrometer (INMS). INMS measures ion and neutral molecules with masses from 2 to 99 (hydrogen, H2, to a little bigger than toluene, C7H8). Pre-Cassini models of the chemistry in Titan's atmosphere did a pretty good job of reproducing the stratospheric composition measurements from Voyager, but it turns out that models of the chemistry really missed something truly fundamental about Titan's chemistry.
Before Cassini, we believed for the most part that the "species" (word to encompass both ions and neutral molecules) were composed only of carbon and hydrogen. We knew there was probably some nitrogen in the smaller molecules, since Voyager observed HCN. What we didn't realize is how much nitrogen participates in the chemistry. Nitrogen's presence results in the formation of a number of molecules and ions that weren't even included in the pre-Cassini models. We care about nitrogen because nitrogen is very important for life on Earth and we are trying to understand what kind of molecules can form in an atmosphere and whether they might play a role in the origin and evolution of life in the Universe.
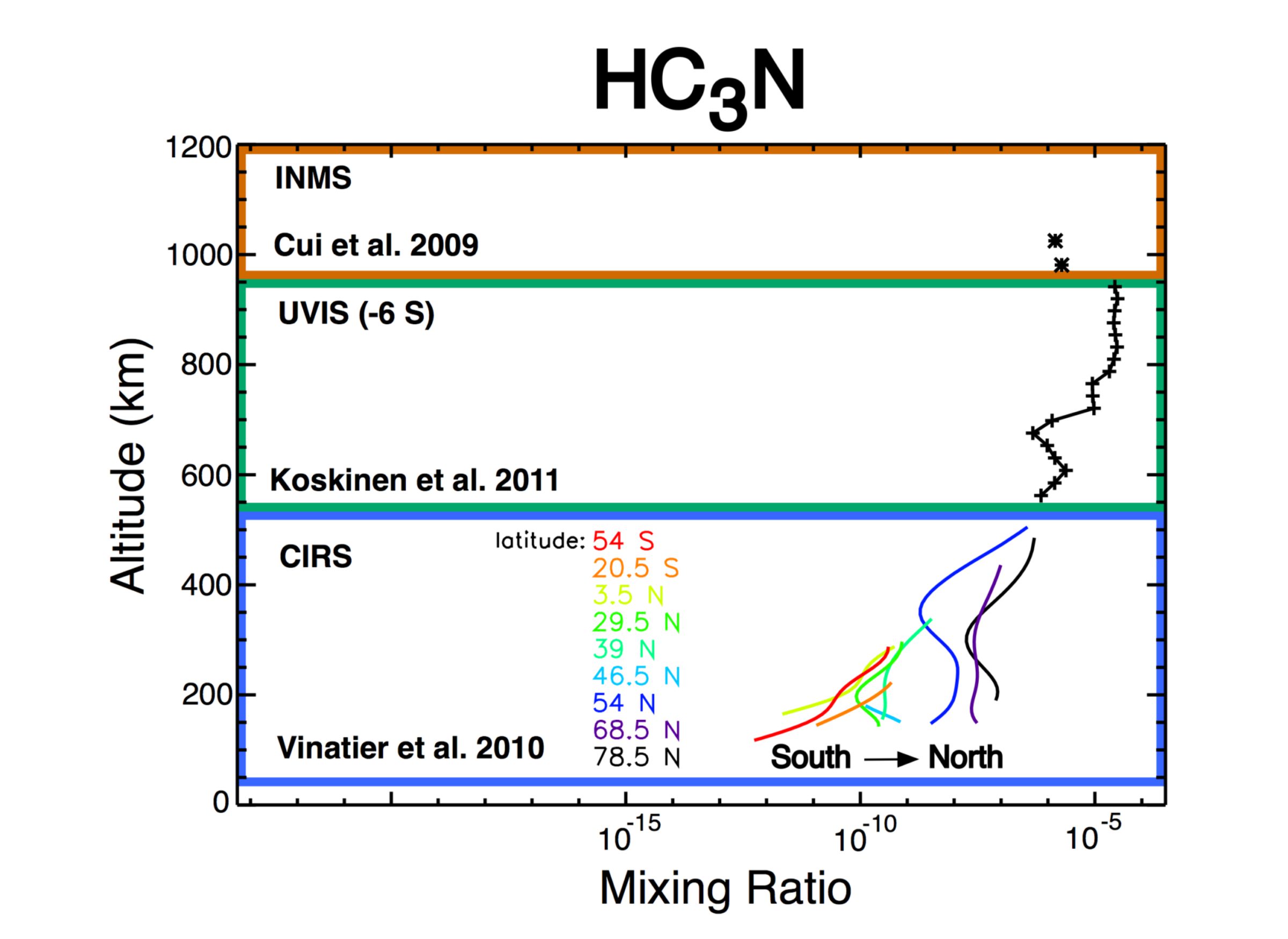
Furthermore, we didn't appreciate how heavy the molecules and ions would be (this is, as I mentioned before, a huge understatement). In the early days of the mission INMS was supposed to be able to measure heavier molecules and ions. The mass range was decreased due to budget constraints. My PhD advisor says he remembers people arguing that even 100 was too high and that there would not be any signal above a mass of 50. Wow. We were so wrong (and here I am using the royal we because I was still in high school when Cassini launched...so it isn't my fault). What we do know now, thanks to both INMS and CAPS, is that Titan has the most complex and chemically diverse ionosphere in the solar system. And that makes one thing very, very clear: if we want to understand organic chemistry in an atmosphere, we have to study Titan. We have to go back to Titan.
A little over a month ago, our friend and colleague Hasso Niemann passed away. Hasso was the principal investigator (PI) of the GCMS carried by Huygens and was instrumental in the construction of the Ion and Neutral Mass Spectrometer as well. (Not to mention mass spectrometers sent elsewhere in the solar system like the one carried by the Galileo Probe.) To say that Hasso revolutionized mass spectrometry in the solar system would be an understatement. Words could never express how grateful I am to Hasso for the beautiful measurements we have of Titan's atmosphere, which have opened our imaginations to a level of atmospheric chemistry complexity we never dreamed of. It is difficult to describe to you how amazing these measurements are but I hope that I have given you at least a small window into Hasso's incredible gift to us. One of my favorite quotations comes from Galileo: "Measure what is measureable and make measurable what cannot now be measured." Thank you so much Hasso, for making measurable what we could not measure before Cassini-Huygens.
It took me longer to finish this post than I had intended and I just learned of the passing of another wonderful colleague, Bishun Khare. Bishun worked with Carl Sagan for many years and together they coined the word "tholin." Bishun's tireless and meticulous laboratory studies form the basis for much of the analysis of haze measurements from Cassini-Huygens (and are used today in the analysis of remote sensing of almost every hazy atmosphere including exoplanets). At DPS in 2010, Bishun and I had adjacent posters and his kindness and excitement about my work, though I was just a student, touched my heart in a way that I am only now beginning to understand. He will be greatly missed.
Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

