Emily Lakdawalla • Jul 30, 2018
Curiosity's organics on Mars
Last month, Curiosity mission scientists published a pair of papers in Science magazine. One of them was about methane, and the other was about organic materials in Mars rocks. There was a lot of hype and speculation around the announcement, and some good and some not-so-good journalism. I thought I could help matters by translating one of the papers from "scientist-ese" into terms that a broader group could understand. I decided to do the organics paper, by Jen Eigenbrode et al., because the word "organics" makes a lot of people jump to (incorrect) conclusions about life on Mars.
The following is a fairly direct translation of the paper, though I have added in additional context to support your understanding. I put that additional contextual text in italics to separate it from the more strictly translated material. This blog post is mostly in the same order as the original paper until I get to the discussion section, where I rearranged things to help readers follow the argument.

Introduction
What is "organic matter" and why is Curiosity looking for it? Organic matter refers to carbon-containing compounds. On Earth, most organic matter comes from life, but there is a lot of carbon in the solar system that did not originate from life.
We are interested in the organic matter in Martian materials for several reasons. It could represent the remains of ancient life. It could serve as food for present-day life. It could, like organic matter in the rest of the solar system, have nothing to do with life. Regardless, if we can find organic compounds in rocks, we can learn a lot about the chemistry of the conditions that existed when the rocks formed and as they were altered over time.
Curiosity has an instrument that is specifically designed to look for organic materials in Martian soils and rocks. Curiosity feeds soil or powdered rock to its Sample Analysis at Mars (SAM) instrument and uses a variety of techniques to figure out whether it contains organics, and what kinds of materials they are.
Because most people think "life" when they hear "organic," I prefer to use terms like "carbon-containing compounds," which is what I'll do for the rest of this article.
SAM has already detected carbon-containing compounds that include a lot of chlorine, compounds like chlorobenzenes and dichloroalkanes. Once we saw these results, we checked the archival data from a similar instrument that operated on Viking, and saw weak evidence for chlorine-containing carbon compounds there, too. Other scientists have examined Mars meteorites and found carbon-containing compounds that came from deep within the planet. These results are all interesting, but they don't tell us much about what these carbon compounds were doing on ancient Mars.
We are especially interested in watery ancient Mars environments, which are what Curiosity was sent to Mars to study. We are checking out sedimentary rocks in Gale crater that record evidence for ancient lakes and rivers.
Sedimentary rocks are rocks made of broken-up bits of other rocks. Sedimentary rocks can form when wind or water or other forces break up and move sediment around. Looking at the shapes of grains in sedimentary rocks, or how they are layered, can tell us whether their sediments were originally deposited by wind, which would be less interesting in terms of the potential for life on Mars, or by water, which would be more interesting.
At the base of Aeolis Mons in Gale crater, we've been exploring a rock that we've named the Murray formation, after Bruce Murray. We think the sediments that make up the Murray formation started out as deposits in a long-lasting lake. The lake had pleasant chemistry, not acidic, either neutral or a little alkaline.

The ancient lake within Gale crater was fed by streams that drained off the Gale crater’s rim and central mountain. The streams brought in lots of sediment. Mars is mostly basalt and the streams didn't travel very far, so it's not surprising that the composition of the sediment is like basalt (with minerals like olivine, pyroxene, and feldspar), plus a few other interesting things (clays, sulfates, iron oxides, and some other stuff we're not sure about). The pleasant chemistry and lake environment would've been perfect for collecting, concentrating, and preserving carbon-containing material in rocks that formed 3.5 billion years ago.
This, by the way, is similar in age to the oldest evidence for life we have discovered on Earth. But because Mars' history is less dynamic than Earth's, its ancient rocks are less altered by heat and pressure. Even if life never did start on Mars, these rocks can tell us about the conditions that existed when life was getting its start on Earth, because the two planets would've been much more similar at that time.

How SAM and its experiments work
SAM is a complicated instrument. The particular kind of experiment performed in this study is called Evolved Gas Analysis, or EGA. It begins when Curiosity drills into a rock and gets a sample. The rover delivers a portion of the sample into SAM. The sample falls into a tiny cup made of quartz. SAM has a carousel full of these cups. SAM rotates the carousel and lifts the cup with the sample into an oven. The oven slowly heats the sample, raising its temperature by 35 degrees Celsius each minute. The heat helps break bonds in molecules in the sample, releasing smaller molecules as gases. (The technical term for this is "pyrolysis.") As an example, if there is a carbonate mineral in the sample, the heat will break it down to release carbon dioxide gas. SAM continues raising the temperature until it reaches 860 degrees Celsius.
SAM uses tiny puffs of helium gas to move the gases from the sample cup into a mass spectrometer, which ionizes the molecules and then measures their masses. (Technically, it measures their mass-to-charge ratio.) By measuring what masses of molecules are released at what temperatures, SAM scientists can reason backward to what materials they think were initially present in the sample. Then they run an experiment in a lab on Earth, using a test sample, to see if it releases the same gases at the same temperatures as they observed in the experiment on Mars. This research is time-consuming.
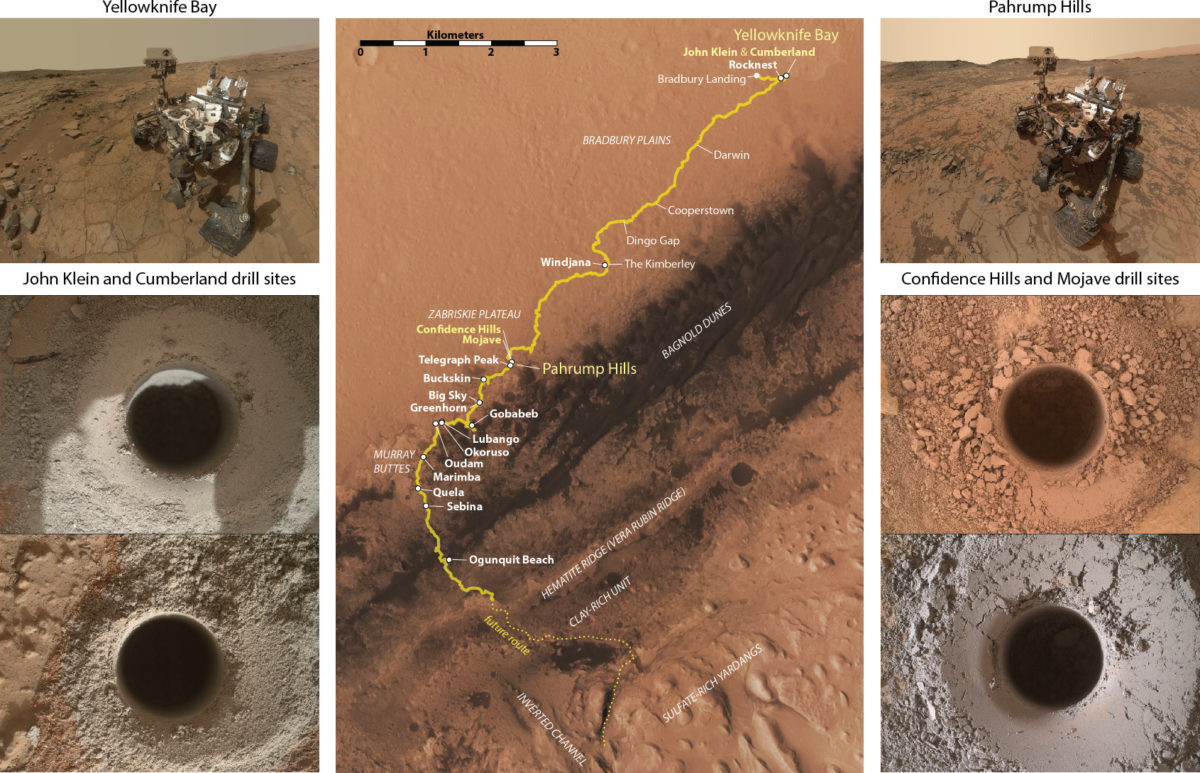
In this paper, we're discussing two drilled samples in particular, Mojave and Confidence Hills. Both of these spots were in the Pahrump Hills, at the very base of the Murray formation. Confidence Hills was drilled on sol 759 (24 Sep 2014), and Mojave on sol 882 (29 Jan 2015). We'll compare these two sample sites with ones from the Sheepbed unit in Yellowknife Bay (the John Klein and Cumberland drill sites, drilled on sols 182 and 279).
Results
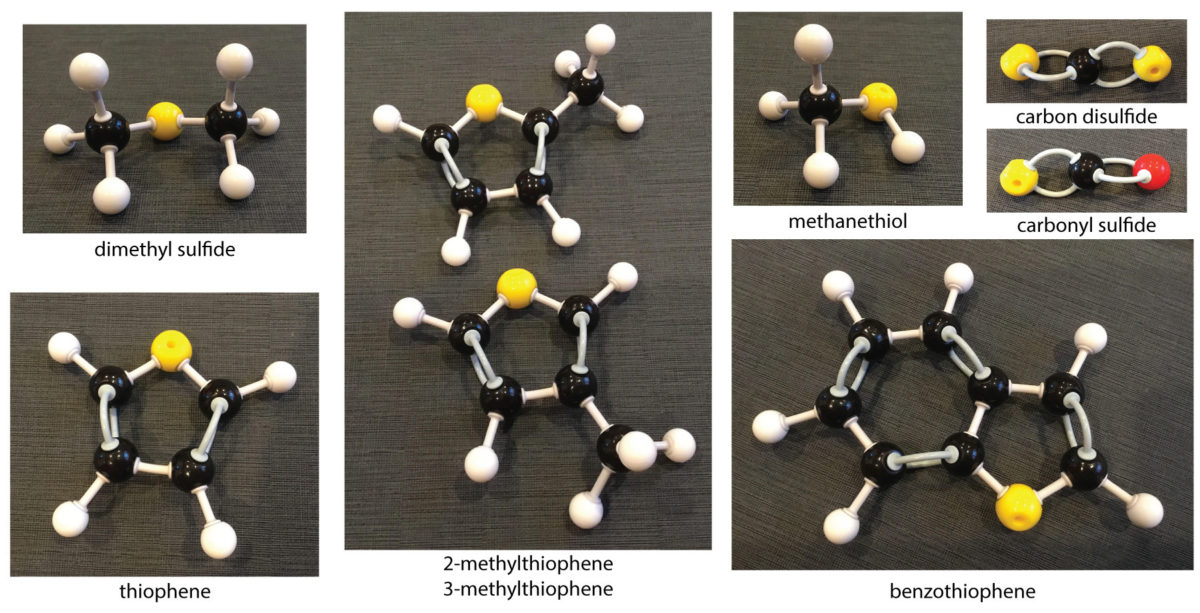
The exciting thing that we found in our mass spec experiment on both Confidence Hills and Mojave was sulfur-containing organic compounds. I'll explain why sulfur is so exciting below. The names of these chemicals are a mouthful:
- thiophene (C4H4S)
- methylthiophenes (C5H6S)
- methanethiol (CH4S)
- dimethyl sulfide (C2H6S)
- maybe benzothiophene (C8H6S)
We saw lots of other carbon-containing compounds without sulfur, too:
- benzene (C6H6)
- toluene (or tropylium ion C7H7 +)
- alkylbenzenes (C8H9 or benzoate ion C7H5O−)
- chlorobenzene (C6H5Cl)
- naphthalene (C10H8)
- lots of carbon-chain molecules with 1 to 5 carbons in them
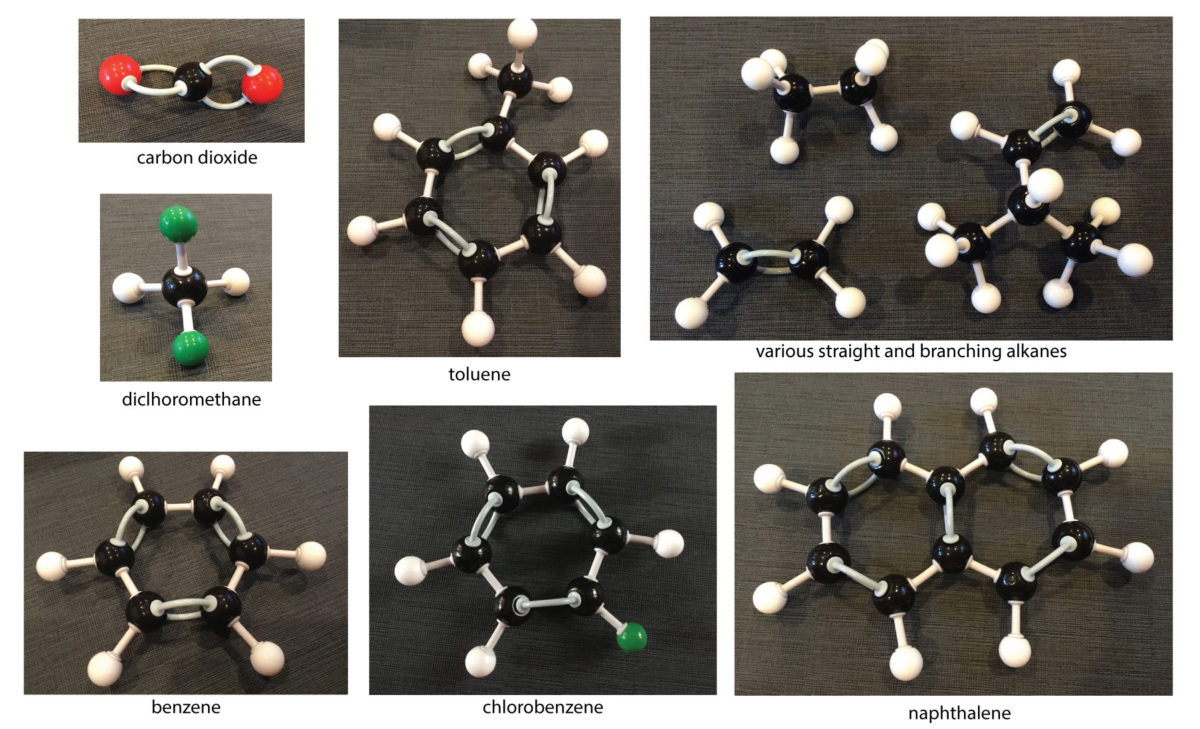
Finally, we saw a lot of smaller molecules:
- carbonyl sulfide (COS)
- carbon disulfide (CS2)
- hydrogen sulfide (H2S)
- sulfur dioxide (SO2)
- oxygen (O2)
- carbon monoxide (CO)
- carbon dioxide (CO2)
We can calculate the total organic carbon present in the samples in SAM, and come up with an estimate of 10 to 100 nanomoles. (If "nanomoles" is meaningless to you, read it as "a very small but present amount.") However, this is just a lower limit, because SAM can't heat its samples hot enough to release all the carbon; at least some is undoubtedly still in the sample cup.
For reasons you can read in the full article, we are sure that the diverse array of compounds that we saw when we heated our sample to high temperature did not come from inside our instrument or from any kind of contamination.
Interpretation
Based on all the evidence, here is our preferred interpretation of the results. We have to reason backward from the wide variety of materials that we saw to figure out what was present in the cup when we started heating it.
The variety of different carbon-containing compounds that is present is strong evidence that they broke off of much bigger molecules. We think the Mojave and Confidence Hills sample in the cup started out with large organic molecules that are ancient -- that is, the big molecules were there when the lake was a lake.
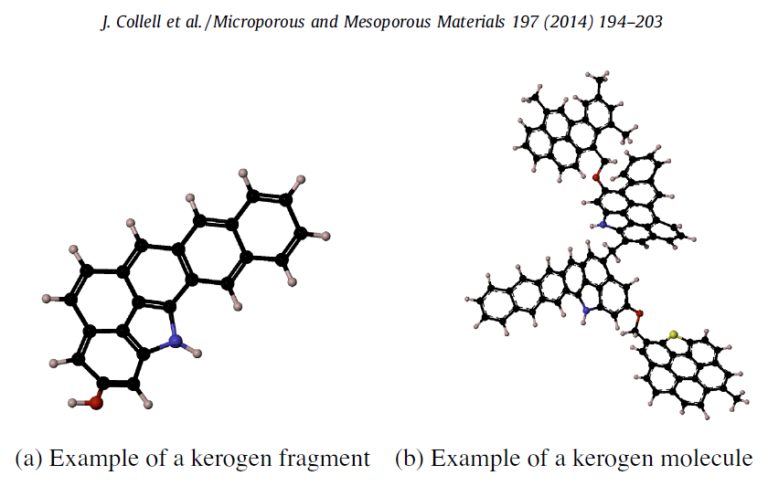
They were big molecules with lots of carbons in rings and chains, called kerogens. Kerogens are a type of molecule that scientists have observed in carbon-rich meteorites, in ancient Earth rocks, and coals. They are made of lots of disorderly-looking linked rings and chains of carbon atoms. When SAM heated these kerogens, we think, single carbon rings and short chains broke off; we can't detect kerogens directly, but we can see their broken bits in SAM evolved gas analysis.
Discussion
What made the kerogens that SAM found in Pahrump Hills rocks?
Biology is one possibility, but the kerogens could also have come from interplanetary dust particles or even igneous rocks (rocks that solidified from a melt).
We decided to test some analogous materials in an Earth copy of the SAM instrument to see if they released collections of smaller carbon-containing compounds that are similar to the collection we observed with SAM on Mars. We ran our experiment on a small sample of the Murchison meteorite (which has a composition similar to interplanetary dust particles) and the Tissint Mars meteorite (which has an igneous origin). Both of these do contain kerogens. Both of these experiments produced carbon-containing compounds like the ones we saw in the Mojave and Confidence Hills samples from Mars, although each rock (Curiosity sample, Murchison, and Tissint) produced different amounts of the different kinds of compounds. So these additional experiments didn't tell us anything about where the carbon compounds we detected on Mars came from, but they do support our favored interpretation, that the Mars rocks contained kerogens.
We wish we could tell you where these large carbon molecules came from. We can't. All options -- biology, Mars geology, meteorites -- are on the table. If biology was a factor, then a lot has happened to these organic molecules over time; there's nothing in what we saw with SAM that makes biology a better interpretation than any other.
Why didn't we see the same materials at Yellowknife Bay that we saw at Pahrump Hills?
We're not sure why the Yellowknife Bay rocks didn't release the same kinds of carbon compounds under the same experimental conditions. Maybe they had less kerogen material to begin with. Maybe the water alteration they experienced a long time ago destroyed the kerogens. Maybe they have been exposed to the harsh Martian environment for too long, and any kerogen molecules that they contained have already broken down. We do know from previous experiments that the Yellowknife Bay mudstones have been exposed at the surface for about 80 million years; maybe the Pahrump Hills rocks have not been exposed for so long, and their kerogens have survived to the present day.
It's actually surprising that the Pahrump Hills rocks preserved kerogens in them for so long. Other work suggests acid fluids circulated through the rocks from the top down about 2.1 billion years ago. We'd ordinarily expect acid fluids to attack large carbon-containing molecules (by oxidizing them and breaking them into tiny molecules like carbon dioxide), so we have to come up with an explanation for how the molecules were preserved for long enough for us to find them.
We can go through a list of possibilities, but we believe that the most important preservation agent was the presence of sulfur in the molecules. The Mojave and Confidence Hills SAM experiment yielded much more carbon bound to sulfur in a variety of different compounds than any other experiments we've done on Mars. The sulfur atoms help link up smaller carbon-containing molecules into much bigger ones, and then prevent them from breaking down. We take advantage of this property of sulfur industrially on Earth to help carbon-containing molecules stay big and unbroken. It's vulcanization, the same process that tire manufacturers use to keep tire rubber from degrading. When those big sulfur-containing carbon-rich molecules get attacked by oxygen, the oxygen is more likely to react with and break off the sulfur, leaving the carbon behind, preserving the big molecules.
The kerogen molecules in the Pahrump Hills rocks could've had this sulfur from their beginnings (whatever their origin), but it's also possible that the molecules didn't start out containing so much sulfur. Sulfur could have come in at the time that the lakebed sediments were turning into rock. In fact, we have evidence from SAM that hydrothermal groundwater that contained sulfides once percolated through these particular rocks. There was probably hydrogen sulfide present, which would've reacted with the carbon-containing molecules to leave behind a sulfur atom in the molecular structure.
Is there anything we can learn about the potential for life on ancient Mars from this study?
When the Curiosity team announced the discovery of a habitable environment preserved in the Sheepbed mudstones, the environment that they discussed is one that could be inhabited by chemolithoautotrophs. (It's a terrifying word but you can sound it out if you go slowly. Chemo, litho, auto, trophs.) Chemolithoautotrophs are organisms that get their carbon from carbon dioxide -- they can build themselves out of carbon that doesn't have a biological origin. But the fact that we've observed large carbon-containing molecules preserved at Pahrump Hills tells us that the Mars lakes could also have supported heterotrophs. Heterotrophs are organisms that build themselves out of organic carbon (that is, big carbon molecules, regardless of their origin). The presence of kerogens preserved from ancient Mars tells us that the environment in which the Pahrump Hills sediments were laid down was a more habitable environment than we've talked about in the past.
While it's disappointing that we can't figure out where the carbon-rich large molecules came from, it's really important that we were able to detect them in Mars material drilled from just beneath the surface. Mars' present-day environment is a very harsh one in which large carbon-containing molecules would be expected to degrade rapidly. If we can dig a little deeper, and look in the right places, we now have evidence-based hope that we can find better-preserved molecules in Mars rocks. At a site selected for better preservation of ancient materials, we just may be able to determine whether these molecules came from space, from igneous rocks, from hydrothermal activity, or -- the most exciting possibility -- ancient Mars life.
Thanks to Jen Eigenbrode for reading this post and offering corrections.
Support our core enterprises
Your support powers our mission to explore worlds, find life, and defend Earth. You make all the difference when you make a gift. Give today!
Donate

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

